Abstract
Matrix metalloproteinases (MMPs) may limit severely the pulmonary vasodilatory and inotropic effects of adrenomedullin during pulmonary hypertension. While doxycycline inhibits MMPs and prevents the hemodynamic disorders associated with acute pulmonary embolism (APE), no previous study evaluated if doxycycline enhances adrenomedullin-induced pulmonary vasodilation and contributes to the attenuation of APE-induced pulmonary hypertension. Hemodynamic and respiratory changes were determined in animals not subjected to any intervention (Sham group), or animals subjected to APE induced by microspheres treated with saline (PE group) or 10 mg/kg of doxycycline (Dox+PE group) 30 min before APE or 50 ng/kg/min of adrenomedullin (PE+Adm group) 30 after APE, or doxycycline combined with adrenomedullin (Dox+PE+Adm group). Doxycycline produced no effect on significant temporal decreases in pulmonary vascular resistance index and increases in cardiac index (both by 25%) observed with adrenomedullin. Adrenomedullin produced moderate systemic hypotension. Significant decreases in arterial oxygen partial pressure were observed after doxycycline or APE, but these changes were not affected by adrenomedullin. These results show that the combined administration of doxycycline and adrenomedullin is not advantageous compared with adrenomedullin alone, thus suggesting that adrenomedullin counteracted the pulmonary vasoconstriction and it may help in the therapy of the detrimental acute hemodynamic consequences of APE.
Keywords
adrenomedullin, matrix metalloproteinases, pulmonary embolism, pulmonary hypertension
Introduction
Pulmonary circulation has a key role in filtering thrombi present in the deep venous system, which are the most common source of embolus to the lungs, causing acute pulmonary embolism (APE). APE-induced pulmonary hypertension leading to acute right heart failure and circulatory shock are important causes of morbidity and death [1-3].
The usual therapy of APE includes supportive care, systemic anticoagulation, systemic thrombolysis and surgical embolectomy [4].While the current treatment of APE targets the mechanical obstruction, recent studies have highlighted the relevance of pulmonary arterial vasoconstriction immediately after APE is installed [5]. However, few studies have addressed the hypothesis that the combination of drugs that attenuate the pulmonary hypertension and that improve the cardiac output during APE may produce complementary and beneficial effects than might enhance survival of APE [6].
Previous study demonstrated that adrenomedullin (50 ng/kg/min) intravenously administrated induced a long-lasting reduction of pulmonary vascular resistance and improved cardiac index (both by 50%) after pulmonary hypertension induced by endotoxin in sheep [7]. Furthermore, the same dose of adrenomedullin (for 30 minutes) in patients with pulmonary hypertension greatly increased cardiac index by 44% and that resulted in a 32% decrease in pulmonary vascular resistance[8-10]. Recently, we also showed adrenomedullin significantly decreased pulmonary vascular resistance and increased cardiac index, both by 25% [11] after APE-induced pulmonary hypertension in anaesthetized sheep. Notably, adrenomedullin treatment resulted in a significant attenuation of pulmonary hypertension without impacting on systemic blood pressure, heart rate and global oxygen transport[7,9,11]. Therefore, it appears that the current literature supports the hypothesis that adrenomedullin may be a therapeutic agent with inotropic and pulmonary vasodilatory effects after APE, being a promising adjunct in the therapeutic approach targeting the treatment of acute pulmonary hypertension, independent of its pathogenesis [8,12-14].
Interestingly, previous studies have shown that adrenomedullin may be degraded by matrix metalloproteinases (MMPs), resulting in smaller peptides that promote vasoconstriction, thereby inhibiting the hypotensive effect adrenomedullin dependent [15]. Accordingly, the MMPs inhibition with doxycycline prevented both decreases in circulating adrenomedullin and hypertension in rats [16]. Additionally, the up-regulation of MMPs has also been involved in the development of APE-induced pulmonary hypertension [17-19], since the MMPs inhibition with doxycycline produced beneficial effects on the hemodynamic derangements associated with APE [20]. Supporting these findings, the activation of MMPs in pulmonary vessels and in the right ventricle may contribute to the pulmonary vasoconstriction and to the decreased inotropic activity in the setting of APE, causing myocardial contractile dysfunction [21-24].
Together, the above-mentioned studies support the hypothesis that increased MMPs activity may be limiting severely the pulmonary vasodilatory and impair the inotropic effects of adrenomedullin during APE. However, no previous study has examined whether doxycycline enhance the pulmonary vasodilatory and positive inotropic effects produced by adrenomedullin during APE [11].
Methods
Animal model and cardiopulmonary hemodynamic measurements
The study complied with guidelines of the Institutional Animal Care Committee (protocol nº 457/2013) and the animals were handled according to the guidelines published by the European Union Directive (2010/63/EU) and the ARRIVE (Animal Research: Reporting of In Vivo Experiments). We used a whole animal model of APE [25] to study the hemodynamic effect of intravenous (i.v.) doxycycline combined with i.v. adrenomedullin on APE-induced hemodynamic changes. Sixteen rams of the Santa Inês breed (36.3 ± 3.1 kg) received fentanyl (5 µg/kg, i.v.) before anesthesia was induced and maintained with ketamine (7.5 mg/kg, i.v. bolus, followed by 20 mg/kg/h, i.v.) and midazolam (0.35 mg/kg bolus i.v., followed by 0.25 mg/kg/h, i.v.). Their lungs were mechanically ventilated with an inspired O2 fraction > 0.9 (Dräger Primus, Drägerwerk AG & Co, Lübeck, Germany) during conditions of neuromuscular blockade produced by atracurium (0.3 mg/kg, followed by 0.5 mg/kg/h, i.v.). The tidal volume and the inspiration-to-expiration ratio were held constant throughout the study (15 ml/kg and 1:2, respectively), while the respiratory rate was adjusted as necessary to maintain eucapnia (PaCO2 between 35 to 45 mmHg).
A 20-gauge catheter (Insyte, Becton Dickinson, Sao Paulo, Brazil) was placed into a cephalic vein for drug injection and Lactated Ringer’s administration (2 mL/kg/h). The femoral artery was catheterized with an 18-gauge for monitoring mean arterial pressure via a fluid-filled pressure transducer system (Tru Wave PX 260, Edward Lifesciences, Irvine, CA) and for collecting samples in heparinized syringes for temperature corrected blood gas analysis (348 pH Blood Gas Analyzer, Siemens, Halstead, UK).
A fluid-filled 7.5F balloon-tipped Swan-Ganz thermodilution catheter (Model 131HF7, Edwards Lifesciences, Irvine, CA) was inserted into the jugular vein through an 8.5 Fr introducer sheath and advanced until its tip reached the pulmonary artery based on the observations of pressure waveforms on the screen of a monitor (AS/3, Datex Ëngstrom, Helsinki, Finland). The proximal and distal ports of the catheter were connected to two pressure transducers to allow monitoring of central venous and pulmonary artery pressures, respectively. The pulmonary artery occlusion pressure was measured by temporarily insufflating the balloon of the catheter with 0.7 ml of air. Transducers were zeroed at the heart level before beginning the hemodynamic assessments.
Cardiac output was measured in triplicate by injecting 5 mL of cold (3-5°C) 5% dextrose solution into the central venous pressure port and heart rate was calculated by the electrocardiogram. Cardiac index, pulmonary and systemic vascular resistance indexes were calculated using standard formulae. Heart rate was monitored by a lead II electrocardiogram.
Arterial blood samples were drawn from the femoral artery catheter for measuring temperature-corrected pH, carbon dioxide partial pressure (PaCO2), and oxygen partial pressure (PaO2) (348 pH Blood Gas Analyzer, Siemens, Halstead, UK).
Study design and data collection
The animals were randomly assigned to two experimental groups (n=8 per group) as follows:
1) Dox+PE group: sheep that received doxycycline (10 mg/kg, i.v. during 10 min; [23]) followed 30 min later by APE induced by injection of silicone microspheres (10 mg/mL, Sephadex G50; Pharmacia Fine Chemicals; Uppsala, Sweden) as described previously [11] and 60 min later an infusion of physiological saline (placebo) maintained for 30 min (Pump 11 Elite, Harvard Apparatus, Holliston, MA).
2) Dox+PE+Adm group: sheep that received doxycycline (same dose as the Dox+PE group) followed 30 min later by APE, and 60 min later by an infusion of adrenomedullin (50 ng/kg/min, i.v. [7]) (Human Adrenomedullin, Bachem AG, Bubendorf, Switzerland) maintained for 30 min (Pump 11 Elite).
Baseline measurements (BL) were recorded and doxycyline was injected 30 minutes before APE induction. Measurements were performed 15 and 30 min after doxycycline injection (Dox15 and Dox30, respectively). Immediately after the Dox30 time point, animals received the microspheres and data was collected 15 and 30 min after induction of pulmonary embolism (PE15and PE30, respectively). In the Dox+PE+Adm group, 30 minutes after induction of APE, an intravenous adrenomedullin infusion (50 ng/kg/min) was maintained for 30 min and data was recorded 15 and 30 min after commencing the adrenomedullin infusion (Adm15 and Adm30 time points, respectively); while in the Dox+PE group, an equal volume of physiological saline was administered as placebo. Post-treatment data was recorded 15 and 30 min after the adrenomedullin or the placebo infusion were stopped (PT15 and PT30, respectively).
To avoid unnecessary use of animals, the data obtained in this study were compared with previously published data from our laboratory performed under the same experimental conditions evaluating the effects of adrenomedullin using the same model of microsphere-induced APE [11]:
1) Sham group, non-embolized sheep that did not receive any intervention;
2) PE group, sheep that underwent APE induced by microspheres, followed 30 min later by placebo.
3) PE+Adm group, animals where a 30 min infusion of adrenomedullin (Human Adrenomedullin, Bachem AG, Bubendorf, Switzerland) was administered 30 min after induction of APE.
In the Sham, PE, and PE+Adm groups data recording were initiated from the moment recorded immediately before induction of APE (Dox30); the remaining data collection times coincided with the time points described previously. Compared to these groups, cardiopulmonary evaluations in the Dox+PEand Dox+PE+Adm groups were performed for an additional 30 and 15 minutes before the APE induction (BLand Dox15, respectively).
After the end of data collection, a lethal dose of sodium thiopental (30 mg/kg) combined with potassium chloride (150 mg/kg) were administered i.v. while the animals were still anesthetized.
Statistical analysis
A Shapiro-Wilk test was applied to verify normality of data distribution. Two-way analysis of variance (ANOVA) for repeated measures (with time and treatment defined as main effects) followed by the Dunnett’s multiple comparisons test (Prism 6.02; Graph Pad, San Diego, CA) for between and within group comparisons. Differences between groups were evaluated in relation to the PE group. Data obtained in within each group were compared with the PE30 time point as a reference. A type I error rate <0.05 was considered statistically significant. All the results are expressed as means ± S.E.M.
Results
Sham animals presented significantly lower pulmonary vascular resistance index and mean pulmonary artery pressure than corresponding values recorded after microsphere injection in the PE group. Doxycycline injection alone increased pulmonary vascular resistance index and mean pulmonary artery pressure and these pulmonary parameters recorded 30 min after doxycycline injection (Dox30) in doxycycline treated groups were significantly higher in comparison to same time point recorded in the PE group, when embolized controls did not receive any treatment (Figure 1).
Figure 1. Hemodynamic variables (mean ± SEM) recorded in anesthetized sheep that received i.v. doxycycline (10 mg/kg) 30 minutes before induction of pulmonary embolism with microspheres and 30 minutes later were treated with physiological saline or adrenomedullin (50 ng/kg/min, during 30 min) in the Dox+PE and Dox+PE+Adm groups, respectively (n=8 per group). Data were recorded at baseline (BL); after 15 min (Dox15) and 30 min (Dox30) of doxycycline administration; after 15 min (PE15) and 30 min (PE30) of microsphere-induced pulmonary embolism; and after 15 min (Adm15) and 30 min (Adm30) of adrenomedullin or saline infusion, and 15 min (PT15) and 30 min (PT30) after the end of adrenomedullin or saline infusion. Data from a previous study performed in anesthetized sheep not treated with doxycycline is presented for comparison (Lagos-Carvajal et al., 2015). Pulmonary embolism was induced by microspheres and 30 minutes later animals were treated with physiological saline or adrenomedullin (same dose regimen) in the PE and PE+Adm groups, respectively. Anesthetized sheep that did not undergo any intervention (Sham group) were included for comparison (n=8 per group).
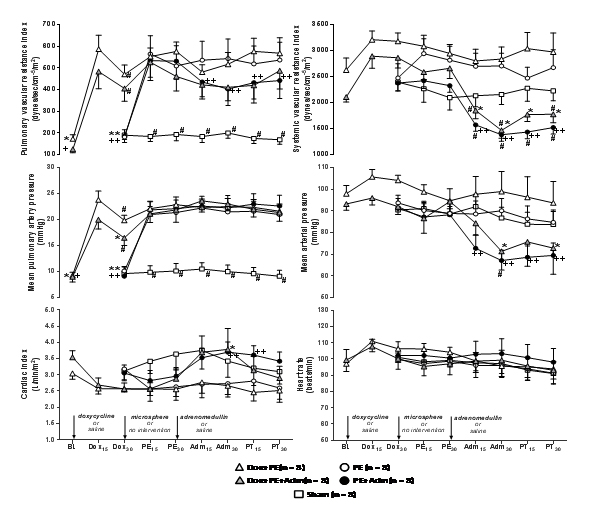
+Significant difference (P<0.05) from PE30 (Dox+PE group);*Significant difference from PE30 (Dox+PE+Adm group);** Significant difference from PE30 (PE Group); ++Significant difference from PE30 (PE+Adm Group); #Significant difference from PE group.
The pulmonary vascular resistance index and mean pulmonary artery pressure values recorded at BL in Dox+PE and Dox+PE+Adm groups were significantly lower than corresponding values recorded 30 min after APE (PE30); while values recorded at Dox15 and Dox30 in both groups that received doxycycline did not differ from PE30, with the exception of the Dox30 time point in the Dox+PE+Adm group, when mean pulmonary artery pressure at Dox30 was 25% lower than the corresponding value recorded at PE30(Figure 1).
Treatment with adrenomedullin significantly decreased pulmonary vascular resistance index and significantly increased cardiac index in comparison to PE30, without causing significant temporal effects on mean pulmonary artery pressure. The Dox+PE and Dox+PE+Adm groups showed no further changes in pulmonary vascular resistance index and mean pulmonary artery pressure in comparison to the PE30 time point. In the PE+Adm and Dox+PE+Adm groups there were no differences in pulmonary vascular resistance index, mean pulmonary artery pressure and cardiac index values recorded after microsphere injection and treatment with adrenomedullin in comparison with values recorded in embolized controls (PE group) (Figure 1).
Doxycycline injection alone did not alter systemic vascular resistance index and mean arterial pressure. Adrenomedullin administration in Dox+PE+Adm and PE+Adm groups induced significant decreases in systemic vascular resistance index from the PE30 time point. This response was accompanied by significant decreases in mean arterial pressure from PE30 in both groups, except for the Adm15 and PT15 time points in the Dox+PE+Adm group. Systemic vascular resistance index values recorded after adrenomedullin administration in Dox+PE+Adm and PE+Adm groups were significantly lower than values recorded in the PE group (except for the PT15 time point in the Dox+PE+Adm group). Mean arterial pressure values were significantly lower in the PE+Adm group than in the PE group only at the Adm30 time point (Figure 1).
Mean PaCO2 values were maintained within physiological limits (35 to 45 mmHg). Small, but statistically significant decreases in PaCO2were recorded at some time points in comparison to the PE30 time point in the Dox+PE group (Dox30and Adm30) and the Dox+PE+Adm group (BL).
The PaO2 recorded 30 min after doxycycline injection (Dox30) in Dox+PE and Dox+PE+Adm groups was significantly higher in comparison to same time point recorded in the PE group (Table 1). In the Dox+PE group, PaO2 was significantly higher at BL and at Dox30 time points in comparison to the PE30 time point. In the Dox+PE+Adm group, PaO2was higher than PE30 only at BL. The PaO2 was significantly lower in the PE group after microsphere injection in comparison to the corresponding time points in the Sham group. After microsphere injection, no significant differences were observed in PaO2 between all other embolized groups and the PE group. Heart rate did not show time related changes and did not differ among five experimental groups throughout the observational period (Figure 1).
|
Parameter
|
Group
|
Time point
|
|
BL
|
Dox30
|
PE30
|
Adm30
|
PT30
|
|
PaCO2
(mmHg)
|
Dox+PE
|
41 ± 1
|
38 ± 1+
|
44 ± 1
|
40 ± 1+
|
42 ± 1
|
|
Dox+PE+Adm
|
40 ± 1*
|
42 ± 1
|
44 ± 1
|
43 ± 1
|
43 ± 1
|
|
PE
|
-
|
42 ± 1
|
43 ± 2
|
43 ± 2
|
41 ± 2
|
|
PE+Adm
|
-
|
42 ± 1
|
41 ± 2
|
39 ± 2
|
41 ± 2
|
|
Sham
|
-
|
43 ± 1
|
42 ± 1
|
41 ± 1
|
40 ± 1
|
|
|
|
|
|
|
|
|
|
PaO2
(mmHg)
|
Dox+PE
|
423 ± 26+
|
234 ± 66+#
|
92 ± 11
|
117 ± 27
|
109 ± 26
|
|
Dox+PE+Adm
|
428 ± 15*
|
149 ± 35#
|
97 ± 22
|
140 ± 38
|
133 ± 34
|
|
PE
|
-
|
390 ± 38**
|
171 ± 33
|
185 ± 33
|
165 ± 23
|
|
PE+Adm
|
-
|
411 ± 30++
|
262 ± 49
|
270 ± 49
|
253 ± 45
|
|
Sham
|
-
|
446 ± 24
|
434 ± 31#
|
449 ± 19#
|
441 ± 16#
|
|
+Significant difference from PE30 (Dox+PE group); *Significant difference from PE30 (Dox+PE+Adm group); **Significant difference from PE30 (PE Group);++Significant difference from PE30 (PE+Adm Group); #Significant difference from PE group
|
Table 1: Arterial carbon dioxide partial pressure (PaCO2) and arterial oxygen partial pressure (PaO2) at recorded in anesthetized sheep that received i.v. doxycycline (10 mg/kg) 30 minutes before induction of pulmonary embolism with microspheres and 30 minutes later were treated with physiological saline or adrenomedullin (50 ng/kg/min, during 30 min) in the Dox+PE and Dox+PE+Adm groups, respectively (n=8 per group). Data were recorded at baseline (BL); after 30 min (Dox30) of doxycycline administration; after 30 min (PE30) of microsphere-induced pulmonary embolism; after 30 min (Adm30) of adrenomedullin or saline infusion, and 30 min (PT30) after the end of adrenomedullin or saline infusion. Data from a previous study performed in anesthetized sheep not treated with doxycycline is presented for comparison [11]. Pulmonary embolism was induced by microspheres and 30 minutes later animals were treated with physiological saline or adrenomedullin (same dose regimen) in the PE and PE+Adm groups, respectively. Anesthetized sheep that did not undergo any intervention (Sham group) were included for comparison (n=8 per group).
Discussion
The main findings of the present study are the combined administration of doxycycline and adrenomedullin is not advantageous compared with adrenomedullin alone, thus suggesting that adrenomedullin counteracted the pulmonary vasoconstriction, reducing the pulmonary vascular resistance and improving the cardiac index, with moderate systemic effects during APE. These results are similar to those previously reported in sheep and humans showing that adrenomedullin induced both pulmonary vasodilation and inotropic positive effect after pulmonary hypertension [7,9]. Therefore, we suggest that the pulmonary vasoconstriction was counteracting by the adrenomedullin and it may help in the therapy of the acute hemodynamic disorder after APE.
Importantly, the suddenly impairment of cardiac output secondary APE, resulting from increased right ventricular afterload imposed by the hypertensive pulmonary circulation, may be responsible by the high incidence of death after APE [3]. In this concern, the significant increases in cardiac index induced by adrenomedullin after APE reported here represent an advantageous action for adrenomedullin in relation to other drugs tested in APE, which are able to attenuate APE-induced pulmonary hypertension by 25%, but not improve cardiac index [22,27].
Although we have not evaluated cAMP le2021 Copyright OAT. All rights reservnamic effects may have been caused by this second messenger, mediating the vasodilatory and positive inotropic responses to adrenomedullin infusion [7-9,11,14,28,29].
We observed no advantageous effect of doxycycline combined with adrenomedullin, neither improving pulmonary vasodilatory nor cardiac index during APE. In fact, although the aim of the present study was not assess the effects of doxycycline many hours after APE, doxycycline produced no changes during early stages (90 min) after APE (Dox+PE group), as previously reported [22,23]. Thus, the absence of attenuation of APE-induced pulmonary hypertension with doxycycline could be related to the period of experimental protocol (90 min of monitoring after the induction of APE) used in this study, i.e., our observational period did not last long enough to evidence such effect, as previously reported that beneficial hemodynamic effects of MMP inhibition with doxycycline were only observed 2 hours after APE was induced [22,23]. Accordingly, an inflammatory response with an early influx of neutrophils and macrophages within the pulmonary artery’s wall showed up only 3 hours after pulmonary embolism in rats [30]. So, the latency necessary to these inflammatory cells can release granules containing large amounts of MMPs (specially the MMP type 9, MMP-9) requests at least 2 or 3 hours after APE is installed. These suggestions may explain the beneficial effects of MMP inhibition with doxycycline have found in previous studies [22,23], but not here. Therefore, our results suggest that combining of doxycycline with adrenomedullin failed to produce synergic effects and is not an appropriated approach in the therapy, at least in the first hour soon after the APE-induced pulmonary hypertension is installed.
The increases in pulmonary vascular resistance index and mean pulmonary artery pressure values (before embolization in the Dox15 and Dox30 time points) observed with doxycycline alone are not related to doxycycline, because the same dose of this drug used here (but it was diluted in saline) produced no deleterious cardiovascular effects when administered intravenously to non-embolized animals [17,22,23]. Supporting our findings, previous studies may explain these apparently conflicting results, which related to the vehicle used to dilution of doxycycline (propylene glycol, the same used here), causing transient increases (peak response) in pulmonary arterial pressure in sheep [31] and fatalities in horses [32]. Importantly, the animals treated with doxycycline also showed decreases in pulmonary oxygenation here, which has not been previously reported [17,22,23]. Since animals were breathing an inspired oxygen fraction > 0.9, the decrease in PaO2 observed in the present study could be attributed to an increase in intrapulmonary shunt (greater percentage of cardiac output towards to non-aerated lung areas). Atelectasis develops during anesthesia in sheep consequently leading to an increase intrapulmonary shunt fraction and oxygenation impairment [33]. Therefore, the decreases in PaO2 recorded after doxycycline might have been attributed drugs’ vehicle (propylene glycol)-induced vasoconstriction in ventilated lung regions leading to the deviation of a fraction of blood flow towards atelectatic lung areas or to vehicles induced pulmonary edema increasing intrapulmonary shunt [31,34].
There are little information regarding to the hemodynamic effects of intravenous infusion of doxycycline [17,22-24, 32], thus, it should be considered that the vehicle used to dilution of doxycycline must be adequately replaced [31,32] or other routes to the administration of doxycycline, such as by via oral[35] must be used to avoid these disadvantageous effects. However, although the mechanisms leading to these conflicting results with doxycycline (an non-specific MMP inhibitor) are not clear at present, the possible therapeutic effects of selective MMP inhibitors should be further examined in APE setting [23], considering the important role played by MMPs hours after the onset of APE.
The present study has some limitations that should be taken into consideration. For example, patients with APE are often managed several hours after the onset of symptoms. Therefore, the very early injection of doxycycline or adrenomedullin would be impossible. Additionally, although adrenomedullin produced beneficial hemodynamic effects after APE (clearly decreased the pulmonary vascular resistance and increased the cardiac index), the sheep model of APE used in the present study (PE group) was not hemodynamically unstable, thus, the moderate systemic vasodilator effect with adrenomedullin could be a reason for concern in patients with massive pulmonary embolism, because the hypotension observed under these circumstances could be aggravated by this peptide. Therefore, adrenomedullin should be used carefully in patients hemodynamically unstable with APE and the concomitant use of vasopressor drugs should be considered to provide hemodynamic stability in hypotensive patients with APE that receive adrenomedullin.
Therefore, we conclude that intravenous administration of doxycycline neither affects APE-induced pulmonary hypertension nor improves the beneficial hemodynamic effects of adrenomedullin. Thus, the combined administration of doxycycline and adrenomedullin is not advantageous compared with adrenomedullin alone, which produced significant increases in cardiac index and decreases in pulmonary vascular resistance, thus, suggesting that adrenomedullin counteracted the pulmonary vasoconstriction and it may be interesting in the therapy of the acute hemodynamic disarrangement of APE.
Acknowledgments
The authors thank “Fundacao de Amparo a Pesquisa do Estado de Sao Paulo” (FAPESP, grant number 2012/12.291-7)” for the financial support. There are no known conflicts of interest.
References
- Stratmann G, Gregory GA (2003) Neurogenic and humoral vasoconstriction in acute pulmonary thromboembolism. Anesth Analg 97: 341-354. [Crossref]
- Wood KE (2011) Major pulmonary embolism. Crit Care Clin 27: 885-906. [Crossref]
- Sanchez O, Trinquart L, Colombet I, Durieux P, Huisman MV, et al. (2008) Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 29: 1569-1577. [Crossref]
- Penaloza A, Roy PM, Kline J (2012) Risk stratification and treatment strategy of pulmonary embolism. Curr Opin Crit Care 18: 318-325. [Crossref]
- Smulders YM (2001) Contribution of pulmonary vasoconstriction to haemodynamic instability after acute pulmonary embolism. Implications for treatment? Neth J Med 58: 241-247.
- Smulders YM (2000) Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 48: 23-33. [Crossref]
- Ertmer C, Morelli A, Rehberg S, Lange M, Hucklenbruch C, et al. (2007) Exogenous adrenomedullin prevents and reverses hypodynamic circulation and pulmonary hypertension in ovine endotoxaemia. Br J Anaesth 99: 830-836.
- Nagaya N, Nishikimi T, Uematsu M, Satoh T, Oya H, et al. (2000) Haemodynamic and hormonal effects of adrenomedullin in patients with pulmonary hypertension. Heart 84: 653-658. [Crossref]
- Nagaya N, Satoh T, Nishikimi T, Uematsu M, Furuichi S, et al. (2000) Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation 101: 498-503.
- Nagaya N, Miyatake K, Kyotani S, Nishikimi T, Nakanishi N, et al. (2003) Pulmonary vasodilator response to adrenomedullin in patients with pulmonary hypertension. Hypertens Res 26: S141-146. [Crossref]
- Lagos-Carvajal AP, Teixeira-Neto FJ, Becerra-Velásquez DR, Diniz MS, Klein AV, et al. (2015) Adrenomedullin induces pulmonar vasodilation but does not attenuate pulmonary hypertension in a sheep modelo of acute pulmonary embolism. Life Sci 139: 139-144.
- Westphal M, Booke M, Dinh-Xuan AT (2004) Adrenomedullin: a smart road from pheochromocytoma to treatment of pulmonary hypertension. Eur Respir J 24: 518-520. [Crossref]
- Nishikimi T, Horio T, Yoshihara F, Nagaya N, Matsuo H, et al. (1998) Effect of adrenomedullin on cAMP and cGMP levels in rat cardiac myocytes and nonmyocytes. Eur J Pharmacol 353: 337-344. [Crossref]
- Rademaker MT, Charles CJ, Lewis LK, Yandle TG, Cooper GJ, et al. (1997) Beneficial hemodynamic and renal effects of adrenomedullin in an ovine model of heart failure. Circulation 96: 1983-1990. [Crossref]
- Martínez A, Oh HR, Unsworth EJ, Bregonzio C, Saavedra JM, et al. (2004) Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J 383. [Crossref]
- Nascimento RA, Mendes G, Possomato-Vieira JS, Gonçalves-Rizzi VH, Kushima H, et al. (2014) Metalloproteinase Inhibition Protects against Reductions in Circulating Adrenomedullin during Lead-induced Acute Hypertension. Basic Clin Pharmacol Toxicol 116: 508-515.
- Fortuna GM, Figueiredo-Lopes L, Dias-Junior CA, Gerlach RF, Tanus-Santos JE (2007) A role for matrix metalloproteinase-9 in the hemodynamic changes following acute pulmonary embolism. Int J Cardiol 114: 22-27. [Crossref]
- Mühl D, Ghosh S, Uzuelli JA, Lantos J, Tanus-Santos JE (2010) Increases in circulating matrix metalloproteinase-9 levels following fibrinolysis for acute pulmonary embolism. Thromb Res 2010; 125: 549-553.
- Cau SB, Barato RC, Celes MR, Muniz JJ, Rossi MA, et al. (2013) Doxycycline prevents acute pulmonary embolism-induced mortality and right ventricular deformation in rats. Cardiovasc Drugs Ther 27: 259-267.
- Neto-Neves EM, Kiss T, Muhl D, Tanus-Santos JE (2013) Matrix metalloproteinases as drug targets in acute pulmonary embolism. Curr Drug Targets 14: 344-352. [Crossref]
- Ali MA, Cho WJ, Hudson B, Kassiri Z, Granzier H, et al. (2010) Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation 122: 2039-2047. [Crossref]
- Neto-Neves EM, Dias-Junior CA, Rizzi E, Castro MM, Sonego F, et al. (2011) Metalloproteinase inhibition protects against cardiomyocyte injury during experimental acute pulmonar thromboembolism. Cri Care Med 39: 349-356.
- Neto-Neves EM, Sousa-Santos O, Ferraz KC, Rizzi E, Ceron CS, et al. (2013) Matrix metalloproteinase inhibition attenuates right ventricular dysfunction and improves responses to dobutamine during acute pulmonary thromboembolism. J Cell Mol Med 17: 1588-1597.
- Ferraz KC, Sousa-Santos O, Neto-Neves EM, Rizzi E, Muniz JJ, et al. (2013) Recombinant human matrix metalloproteinase-2 impairs cardiovascular ß-adrenergic responses. Basic Clin Pharmacol Toxicol 112: 103-109.
- Velásquez DR, Teixeira-Neto FJ, Lagos-Carvajal AP, Steim-Diniz M, Rodríguez NC, et al. (2015) Effects of different inspired oxygen fractions on sildenafil-induced pulmonary anti-hypertensive effects in a sheep model of acute pulmonary embolism. Life Sci 127: 26-31.
- Neto-Neves EM, Dias-Junior CA, Uzuelli JA, Pereira RP, Spiller F, et al. (2011) Sildenafil improves the beneficial hemodynamic effects exerted by atorvastatin during acute pulmonary thromboembolism. Eur J Pharmacol 670: 554-560.
- Dias-Junior CA, Neto-Neves EM, Montenegro MF, Tanus-Santos JE (2010) Hemodynamic effects of inducible nitric oxide synthase inhibition combined with sildenafil during acute pulmonary embolism. Nitric Oxide 23: 284-288.
- Szokodi I, Kinnunen P, Ruskoaho H (1996) Inotropic effect of adrenomedullin in the isolated perfused rat heart. Acta Physiol Scand 156: 151-152. [Crossref]
- Szokodi I, Kinnunen P, Tavi P, Weckström M, Tóth M, et al. (1998) Evidence for cAMP-independent mechanisms mediating the effects of adrenomedullin, a new inotropic peptide. Circulation 97: 1062-1070. [Crossref]
- Eagleton MJ, Henke PK, Luke CE, Hawley AE, Bedi A, et al. (2002) Southern Association for Vascular Surgery William J. von Leibig Award. Inflammation and intimal hyperplasia associated with experimental pulmonary embolism. J Vasc Surg 36: 581-588. [Crossref]
- Quinn DA, Robinson D, Hales CA (1990) Intravenous injection of propylene glycol causes pulmonary hypertension in sheep. J Appl Physiol 68: 1415-1420. [Crossref]
- Riond JL, Riviere JE, Duckett WM, Atkins CE, Jernigan AD, et al. (1992) Cardiovascular effects and fatalities associated with intravenous administration of doxycycline to horses and ponies. Equine Vet J 24: 41-45. [Crossref]
- Hedenstierna G, Lundquist H, Lundh B, Tokics L, Strandberg A, et al. (1989) Pulmonary densities during anaesthesia. An experimental study on lung morphology and gas exchange. Eur Respir J 2: 528-535.
- Pearl RG, Finn JC (1990) Hemodynamic effects of diltiazem during vasoconstrictor pulmonary hypertension in sheep. Anesth Analg 71: 493-497. [Crossref]
- Tynan BE, Papich MG, Kerl ME, Cohn LA (2015) Pharmacokinetics of minocycline in domestic cats. J Feline Med Surg. [Crossref]

