Caffeine (1,3,7-trimethylxantine) is the most commonly consumed social drug in western society for increased physical and cognitive performance [1-5], and it is the main ergogenic resource used by athletes [6]. Initially controversial, caffeine was banned by the International Olympic Committee from 1980-2003 [1,6,7], but then in 2004 its use was approved by the World Anti-doping Agency (WADA), and later this year by the U.S. Anti-doping Agency (USADA; 2016). However, caffeine has remained on the lists of monitored substances of these anti-doping agencies.
The ergogenic effects of caffeine have been demonstrated in different sport modalities, namely running [8,9], cycling [10,11], rowing [12], track and field [3], team sports [4], and martial arts [13], among others.
Caffeine is rapidly and completely absorbed by the gastrointestinal tract and is readily distributed throughout all tissues of the body, including muscles and the central nervous system (CNS), which are believed to be the main recipients of caffeine’s ergogenic effects [2,14]. Ergogenic doses of caffeine ranging from 3 to 9 mg/kg body mass [6] appear to have no adverse effects. A moderate oral dose of 6 mg/kg body mass, which elicits peak plasma levels of about 60 µmol/L concentrations after 30 to 60 min, with half-life for elimination range between 2.5–10 h, is known to enhance physical and cognitive performance [5,6,10,14,15]. Blood levels of 1 – 2 mmol/L are known to be toxic and even lethal [14], and have been associated with suicides [16]. Caffeine’s molecular mechanisms for increasing physical performance are still virtually undefined. However, due to its ability to cross the blood-brain barrier at blood concentrations generated by a moderate ergogenic dose, and because of its properties as a stimulant psychotropic drug [15,17], mechanisms involving metabolic and central effects have been proposed.
Metabolic effects of caffeine have been mainly related to the enhancement of lipolysis, fatty acid oxidation and energy expenditure via the stimulation of the sympathetic nervous system [18,19] and a sequential sparing of muscle glycogen [20]. However, the main pharmacological effects of caffeine appear to be mediated via the CNS where caffeine counterbalances the inhibitory neuromodulation of adenosine in order to induce effects on both the CNS and peripheral nervous system to reduce pain and exertion perception [21], to improve motor recruitment [22] and to increase excitation-contraction coupling [23,24].
Caffeine is a non-selective competitive adenosine receptor antagonist (A1R and A2AR subtypes) that increases neurotransmission via dopamine D2 receptors (D2R) [25,26]. The striatum expresses high levels of A2AR where they are co-expressed with postsynaptic D2R, forming A2AR-D2R heterodimers [25]. In this scenario, caffeine fails to be ergogenic in the mouse lacking A2AR [27], or in wild type rats treated with the selective A2AR agonist 5'-N-ethylcarboxamidoadenosine (NECA) [26], which denotes the participation of these receptors in caffeine’s central-mediated ergogenic effects.
When designing experimental models to better define the molecular mechanisms involved in the enhanced capacity of caffeine to positively modulate physical performance, animals received the drug mostly during resting metabolic conditions. Here, we confirmed previous observations that a moderate human ergogenic dose of caffeine (6 mg / kg body mass), administered intraperitoneally (i.p.) at resting basal conditions (Figure 1A), significantly increased exercise performance in adult (three-month-old) and older (seven-month-old) mice that were challenged in a maximal exercise treadmill test (Figures 1B and 1D, respectively). Mice were tested in the treadmill after 30 min of caffeine i.p. administration, at the caffeine blood peak period defined for humans [15]. Drug-receiving mice remained on the treadmill for longer periods of time, and as a consequence reaching significantly higher running speeds (Figures 1B and 1D). In addition, higher levels of blood lactate were observed at the end of the exercise protocol, in agreement with increased exercise workloads (Figures 1C for three-month-old mice and Figure 1E for seven-month-old mice). Even when significant, the observed ergogenic effect in both groups of animals was modest compared to the overt effect seen in humans [8,9]. Therefore, we wonder whether the mice were effectively in the blood peak of caffeine and whether it differs when the sympathetic nervous system is stimulated by physical activity [28].
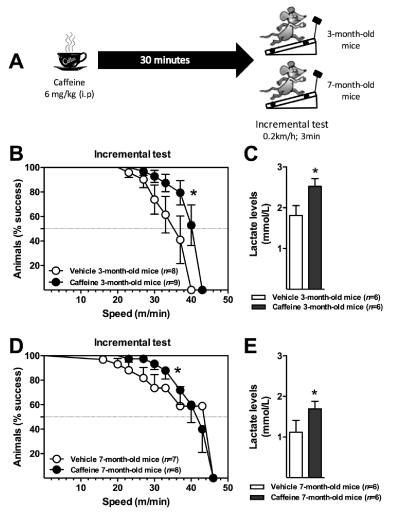
Figure 1. C57BL/6 mice (3- or 7-month-old; male) received a single dose of 6 mg/kg caffeine intraperitoneally (i.p.), and 30 min later animals were submitted to an incremental exercise test (0.2 km/h of increment each 3 min) (A). The ergogenic effect of caffeine was quantified through survival curves (B, D) and by the levels of blood lactate at the end of the protocol (C, E). Lactate concentrations were measured by using a specific sensor (YSI, Yellow Springs, OH, USA). The survival curves were analyzed by the Gehan-Breslow-Wilcoxon test and lactate levels by the Student t test. *P < 0.05. Results are presented as mean ± S.E.M.
Mice at rest or submitted to continuous physical activity for 30 min received a single caffeine dose (6 mg/kg body mass; i.p.) and afterwards the blood and tissue concentrations of the drug were measured over time (Figure 2A). Blood caffeine pharmacokinetics showed the same profile in both groups of mice, in the resting and active animals, with peak plasma levels of 24.1 and 20.0 µmol/L after 15 min, and with a half-life for elimination of 31.6 and 33.5 min, respectively. A single Student t test analysis at 0 and 15 min (Figure 2B) showed statistical differences, indicating the distribution of caffeine into the tissues was faster when the metabolism was activated by physical exercise. In agreement, higher drug concentrations were induced in the muscles that performed the imposed physical activity. Significantly higher concentrations of caffeine were observed in the quadriceps of active mice, while no differences were noticed in the less-recruited, and therefore, less-perfused anterior tibial muscle (Figure 1D). In contrast, lower levels of caffeine accumulated in the brain, particularly in the striatum where reductions up to 50% were found (Figure 1G). Furthermore, among the three brain tissue regions investigated, the striatum showed the lowest caffeine accumulation (Figures 1E to 1G).
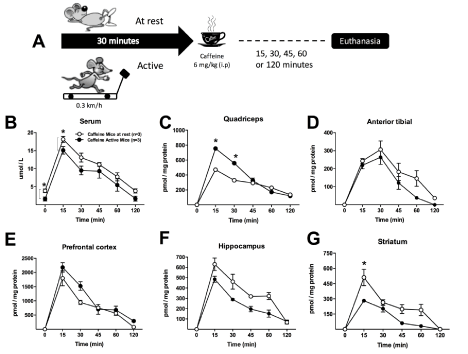
Figure 2. C57BL/6 mice (3-month-old; male) were at rest (mice at rest: white circles) or submitted to 30 minutes of running in an adapted treadmill at a speed of 0.3 km/h (active mice: black circles). After 30 minutes, a single dose of 6 mg / kg caffeine was administrated intraperitoneally (i.p.) (n=3 mice/group) (A) and pharmacokinetics and tissue distribution of caffeine investigated. Caffeine was determined by liquid chromatography coupled to a photodiode array detector (Alliance, Waters, Milford, MA, USA) as previously reported in the serum (B), the skeletal muscles, quadriceps (C) and anterior tibial (D), and in the brain tissue regions, prefrontal cortex (E), hippocampus (F) and striatum (G), 0, 15, 30, 45, 60 and 120 minutes after the drug administration. Two-way ANOVA followed by the Turkey’s test; *P < 0.05. In panel B, Student t test was performed at 0, and 15 min. Results are presented as mean ± S.E.M.
This differential pharmacokinetics observed in the mice encourages more detailed studies in the future with more appropriate experimental designs, since caffeine kinetics does not follow the profile observed in humans. Even when caffeine is orally administered in humans, it has been described that this via has a comparable pharmacokinetics with intravenous administration, leading to superimposable plasma curves [15]. Therefore, if human caffeine pharmacokinetics are directly extrapolated onto different animal models, that will generate contradictory results. The peak of caffeine in the blood is shorter than in humans (about 15 min for mice; about 30 - 60 min for humans), which is in agreement with the greater metabolic rate per gram of body weight observed in mice.
When the sympathetic nervous system, and therefore the metabolism is activated by physical exercise, much attention should also be paid to the group of skeletal muscles involved; i.e. different muscles are recruited in the running wheel, treadmill, swimming pool, etc.; therefore, according to the data presented here, it can be assumed that caffeine distribution will deviate to the more active tissues. Consequently, it is appropriate to perform a caffeine distribution curve for the type of physical activity selected, since the enhancement of lipolysis or fatty acids metabolism could differ according to the levels of caffeine reached, and their corresponding ergogenic effects. In line, it has already been described that high levels of tissue caffeine increases lipolysis and heat production in skeletal muscles by the inhibition of phosphodiesterases and also by increasing the content UCPs (uncoupling proteins) [29]. The effect might be misunderstood if investigating in a non-specific skeletal muscle.
Finally, the central performance-enhancing effect of caffeine, particularly in the striatum, where it enhances neurotransmission through the antagonism of the adenosine receptors, influencing the dopaminergic system [25,26], would be more sensitive to the caffeine ergogenic dose in the treadmill activity, because the lowest levels of the drug were reached in this tissue (Figure 1G). This is relevant when considering the recent findings showing that caffeine also stimulates dopaminergic neurons in a direct and dose-dependent manner [30]. On the other hand, microdialysis studies showed that dopamine levels rise in the brains of rats after an acute caffeine administration, suggesting a direct effect of the ergogenic compound on dopamine synthesis. This effect was also dependent on the dose of caffeine used, and therefore on the elicited levels of caffeine in the brain tissue [31]. However, these data were contradicted by a report performed on humans, demonstrating that caffeine increases striatal dopamine D2/D3 receptor availability and not dopamine levels at doses that are relevant to human consumption [25]. Nevertheless, regardless of the specific mechanism there is growing evidence pointing to the direct effect of caffeine on the dopaminergic system, which appears to be dose-dependent, and that could be erroneously interpreted according to the experimental design used.
Here we consider some potential pitfalls that could emerge when investigating caffeine’s ergogenic effects in mice. Pharmacokinetics of caffeine is in general performed by researchers of the physical exercise field, in experimental animals under resting conditions, which leads to a similar profile as that of rodents where the sympathetic nervous system was activated. However, the tissue distribution of caffeine significantly differs, achieving higher drug accumulation in the skeletal muscle recruited by the specific activity. Brain tissue distribution is difficult to predict; it was observed here that after 30 min of running on the treadmill the lowest concentrations of a single dose of caffeine were achieved in the striatum. Thus, if caffeine’s effects are concentration-dependent, the pharmacokinetics and distribution of the drug must be investigated in advanced.
Acknowledgments
The authors are grateful to Theodore Griswold for language editing. This work was supported by grants from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil), FAPESC/CNPq (Programa de Apoio a Núcleos de Excelência PRONEX; NENASC Project). Latini A is a CNPq fellow.
References
- Ellender L, Linder MM (2005) Sports pharmacology and ergogenic aids. Prim Care 32: 277-292. [Crossref]
- Meeusen R, Roelands B, Spriet LL (2013) Caffeine, exercise and the brain. Nestle Nutr Inst Workshop Ser 76: 1-12. [Crossref]
- Pasman WJ, van Baak MA, Jeukendrup AE, de Haan A (1995) The effect of different dosages of caffeine on endurance performance time. Int J Sports Med 16: 225-230. [Crossref]
- Schneiker KT, Bishop D, Dawson B, Hackett LP (2006) Effects of caffeine on prolonged intermittent-sprint ability in team-sport athletes. Med Sci Sports Exerc 38: 578-585. [Crossref]
- Thein LA, Thein JM, Landry GL (1995) Ergogenic aids. Phys Ther 75: 426-439. [Crossref]
- Stear SJ, Castell LM, Burke LM, Spriet LL (2010) BJSM reviews: A-Z of nutritional supplements: dietary supplements, sports nutrition foods and ergogenic aids for health and performance Part 6. Br J Sports Med 44: 297-298. [Crossref]
- Chester N, Wojek N (2008) Caffeine consumption amongst British athletes following changes to the 2004 WADA prohibited list. Int J Sports Med 29: 524-528. [Crossref]
- Carr A, Dawson B, Schneiker K, Goodman C, Lay B (2008) Effect of caffeine supplementation on repeated sprint running performance. J Sports Med Phys Fitness 48: 472-478. [Crossref]
2021 Copyright OAT. All rights reserv
- de Poli RA, Miyagi WE, Nakamura FY, Zagatto AM (2016) Caffeine Improved Time to Exhaustion, But Did Not Change Alternative Maximal Accumulated Oxygen Deficit Estimated During a Single Supramaximal Running Bout. Int J Sport Nutr Exerc Metab 1-21. [Crossref]
- Connell CJ, Thompson B, Kuhn G, Claffey MP, Duncan S, et al. (2016) Fatigue related impairments in oculomotor control are prevented by caffeine. Sci Rep 6: 26614. [Crossref]
- Green JM, Olenick A, Eastep C, Winchester L (2016) Caffeine influences cadence at lower but not higher intensity RPE-regulated cycling. Physiol Behav 169: 46-51. [Crossref]
- Bruce CR, Anderson ME, Fraser SF, Stepto NK, Klein R, et al. (2000) Enhancement of 2000-m rowing performance after caffeine ingestion. Med Sci Sports Exerc 32: 1958-1963. [Crossref]
- Santos VG, Santos VR, Felippe LJ, Almeida JW Jr, Bertuzzi R, et al. (2014) Caffeine reduces reaction time and improves performance in simulated-contest of taekwondo. Nutrients 6: 637-649. [Crossref]
- Tallis J, James RS, Cox VM, Duncan MJ (2012) The effect of physiological concentrations of caffeine on the power output of maximally and submaximally stimulated mouse EDL (fast) and soleus (slow) muscle. J Appl Physiol (1985) 112: 64-71. [Crossref]
- Blanchard J, Sawers SJ (1983) The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol 24: 93-98. [Crossref]
- Higdon JV, Frei B (2006) Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr 46: 101-123. [Crossref]
- Elmenhorst D, Meyer PT, Matusch A, Winz OH, Bauer A (2012) Caffeine occupancy of human cerebral A1 adenosine receptors: in vivo quantification with 18F-CPFPX and PET. J Nucl Med 53: 1723-1729. [Crossref]
- Acheson KJ, Gremaud G, Meirim I, Montigon F, Krebs Y, et al. (2004) Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am J Clin Nutr 79: 40-46. [Crossref]
- Butcher RW, Baird CE, Sutherland EW (1968) Effects of lipolytic and antilipolytic substances on adenosine 3',5'-monophosphate levels in isolated fat cells. J Biol Chem 243: 1705-1712. [Crossref]
- Spriet LL, MacLean DA, Dyck DJ, Hultman E, Cederblad G, et al. (1992) Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Am J Physiol 262: E891-898. [Crossref]
- Doherty M, Smith PM (2005) Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scand J Med Sci Sports 15: 69-78. [Crossref]
- Tarnopolsky MA (2008) Effect of caffeine on the neuromuscular system--potential as an ergogenic aid. Appl Physiol Nutr Metab 33: 1284-1289. [Crossref]
- Mohr M, Nielsen JJ, Bangsbo J (2011) Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J Appl Physiol (1985) 111: 1372-1379. [Crossref]
- Tarnopolsky M, Cupido C (2000) Caffeine potentiates low frequency skeletal muscle force in habitual and nonhabitual caffeine consumers. J Appl Physiol (1985) 89: 1719-1724. [Crossref]
- Volkow ND, Wang GJ, Logan J, Alexoff D, Fowler JS, et al. (2015) Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl Psychiatry 5: e549. [Crossref]
- Zheng X, Hasegawa H (2016) Administration of caffeine inhibited adenosine receptor agonist-induced decreases in motor performance, thermoregulation, and brain neurotransmitter release in exercising rats. Pharmacol Biochem Behav 140: 82-89. [Crossref]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, et al. (2011) Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 3: 10067-10075. [Crossref]
- Christensen NJ, Galbo H (1983) Sympathetic nervous activity during exercise. Annu Rev Physiol 45: 139-153. [Crossref]
- Kogure A, Sakane N, Takakura Y, Umekawa T, Yoshioka K, et al. (2002) Effects of caffeine on the uncoupling protein family in obese yellow KK mice. Clin Exp Pharmacol Physiol 29: 391-394. [Crossref]
- Nall AH, Shakhmantsir I, Cichewicz K, Birman S, Hirsh J, et al. (2016) Caffeine promotes wakefulness via dopamine signaling in Drosophila. Sci Rep 6: 20938. [Crossref]
- Solinas M, Ferré S, You ZB, Karcz-Kubicha M, Popoli P, et al. (2002) Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci 22: 6321-6324. [Crossref]


