Department of Hematology and Medical Oncology, Emory University School of Medicine, 1365 C Clifton Road, NE, Atlanta, GA 30322, USA
E-mail :yamamasa1155@yahoo.co.jp
DOI: 10.15761/IMM.1000101
Bone homeostasis is mainly regulated by osteoblasts, osteocytes and osteoclasts. Osteoblasts promote bone formation and osteoclasts stimulate bone resorption. Bone loss with aging is due to decreased osteoblastic bone formation and increased osteoclastic bone resorption. Bone loss with various pathophysiologic states leads to osteoporosis. Nutrition and functional food factors may play a role in the prevention of bone loss. Menaquinone-7 (MK-7), a kind of vitamin K2, has been shown to reveal stimulatory effects on osteoblastic bone formation and suppressive effects on osteoclastic bone resorptionin vitro.MK-7 stimulates protein synthesis (including osteocalcin and other protein molecules) in osteoblastic cells. MK-7 regulates the gene expression of various proteins, which are related to cellular functions in osteblastic and osteoclastic cells. MK-7 suppresses activation of NF-κB signaling pathways in osteoblasts and osteoclasts. Moreover, dietary intake of MK-7 has been shown to reveal potential preventive effects on bone loss in osteoporosis animal models and postmenopausal women. This review will discuss the role of dietary MK-7 in the prevention of osteoporosis.
Bone homeostasis is mainly regulated by osteoblasts, osteocytes and osteoclasts. Osteoblasts promote bone formation and osteoclasts stimulate bone resorption [1]. Numerous pathological processes have been shown to stimulate bone resorption and suppress bone formation, leading to bone loss. Osteoporosis with bone loss is widely recognized as a major public health threat. The most dramatic expression of the disease is represented by bone fractures. Postmenopausal osteoporosis, which is induced by ovarian hormone deficiency in women after menopause, leads to severe bone destruction with increasing age [2]. Moreover, malnutrition or undernutrition is often observed in the elderly, and this nutritional state is more intense in patients with bone fracture than in the general aging population [3]. Deficiency in both micronutrients and macronutrients has been suggested to be important factors affecting on bone fracture in the osteoporotic elderly.
There is growing evidence that nutritional and functional food factors have been demonstrated to regulate bone homeostasis and reveal preventive and restorative effects on bone loss with various pathophysiologic conditions [4,5]. Nutritional factor vitamin K, a fat-soluble vitamin, has been suggested to prevent bone fracture with osteoporosis. Vitamin K was originally identified as an essential cofactor for blood coagulation. Vitamin K is an essential in the posttranslational carboxylation of certain protein-bound glutamate residues of osteocalcin, which are converted into gamma (γ)-carboxy glutamate (Gla) by γ-carboxylase [6]. Osteocalcin is synthesized in osteobalsts. These Gla residues form calcium-binding sites that are essential for the activity of the proteins.
Menaquinone-7 (MK-7) is a kind of vitamin K2, which is greatly contained in fermented soybean (natto in Japanese). MK-7 has been shown to reveal osteogenic effects due to stimulating posteoblastic bone formation and to inhibiting osteoclastic bone resorption. Moreover, MK-7 has been shown to play a role in the prevention of bone loss in animal model for osteoporosis and human subjects [5]. This review will discuss recent advances regarding to the action of MK-7 in bone homeostasis and the role of dietary MK-7 intake in the prevention and treatment of osteoporosis.
Vitamin K is a fat-soluble vitamin. There are three types of vitamin K: vitamin K1, (phylloquinone), vitamin K2, (menaquinone), and vitamin K3, (menadione). Vitamin K1,is a sole compound, but vitamin K2,is a series of vitamers with multiisoprene units (one to four) at the 3-position of the naphthoquinone, which is classified according to the length of their aliphatic side chain and is designated as MK-n,wherenstands for the number of isoprenoid residues in that chain. Chemical structure of vitamin K is shown in Figure 1.
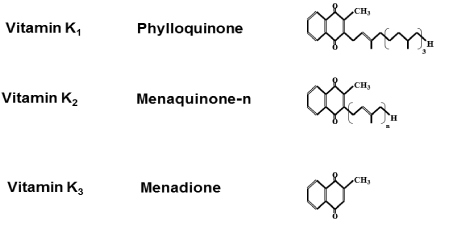
Figure 1. Chemical structure of Vitamin K.
In food, the most important K vitamins are K1,that is found in green vegetables and some plant oils. The long-chain menaquinones (MK) including MK-7, MK-8, and MK-9 are present in fermented foods, notably cheese andnatto.Natto,which is fermented soybeans, is popular food in Japan. Vitamin K1, MK-4 and MK-7 are used in food supplements. Since the molecular structures of vitamin K1,and MK-4 are comparable, both contain 4 isoprenoid residues and 3 of which are saturated in vitamin K1,but contain a double bond in MK- 4. Their physico-chemical characteristics are closely similar (Figure 1). Menaquinones including MK-7 are much more hydrophobic. MK-7 has longer half-life times [7]. MK-7 is incorporated into low-density lipoproteins in the circulation of living body [8]. Vitamin K1 is the most common form of vitamin K in commercially available supplements. MK-7 is used as a supplement.
Bioavailability of MK-4 and MK-7 with nutritional doses has been examined in healthy Japanese women [9]. Serum levels of MK-7 absorbed by intestine were reached to maximal levels at 6 hours after intake, and its levels were detected up to 48 hours after intake with a single dose administration of MK-4 (420 μg; 945 nmol) or MK-7 (420 μg; 647 nmol) [9]. MK-4 was not detectable in the serum of all subjects at any time point, while MK-7 was significantly increased serum MK-7 levels in all subjects [9]. From these findings, it has been assumed that MK-4, which is present in food, may not contribute to the vitamin K status as estimated by serum vitamin K levels [9]. Intestinal absorption of vitamin K from diet gradually was declined with increasing age. Consequently, vitamin K deficiency may occur in elderly.
Vitamin K, an essential factor for blood coagulation, is required for the carboxylation of Gla-proteins in the liver (coagulation factors) and extra-hepatic tissues, such as bone (osteocalcin) and arterial wall (matrix Gla-protein). Vitamin K is an important cofactor for the posttranslational carboxylation of certain protein-bound glutamate residues of osteocalcin, which are converted into γ -carboxy glutamate (Gla) by γ-carboxylase [6,10]. These Gla residues form calcium-binding sites that are essential for the activity of the proteins. During γ –glutamate carboxylation, vitamin K is oxidized into its epoxide form (KO) that is reconverted to vitamin K quinone (K) by the enzyme vitamin K epoxide reductase (VKOR) [11]. Derivatives of 4-hydroxycoumarin (including warfarin and acenocoumarol) specifically inhibit VKOR, which prevents the recycling of vitamin K [11]. In this carboxylation process, glutamate residues are converted into γ-carboxyglutamate (Gla) [10]. Gla residues have a high affinity for calcium, which is an essential property of all Gla proteins. In addition, Gla-containing proteins are known as the blood coagulation factors II, VII, IX, and X, which are all synthesized in the liver [7]. Osteocalcin (synthesized in osteoblast) and matrix Gla protein (primarily synthesized in cartilage and in the vessel wall) are Gla proteins that are not related with blood clotting [7].
Coagulation factors are essential fully carboxylated under normal conditions. Dose–response effects of menaquinone intake on the carboxylation of the extra-hepatic Gla-proteins have been examined [12]. MK-7 supplementation with increasing doses increased the carboxylation of circulating osteocalcin and matrix Gla-protein in forty-two healthy men and women (aged between 18 and 45 years) that were received placebo capsules or MK-7 capsules with a daily dose of 10, 20, 45, 90, 180 or 360 μg [12]. MK-7 intake with nutritional doses improved the carboxylation of the extra-hepatic vitamin K-dependent proteins [12].
Vitamin K has been shown to contribute to bone health through its role as cofactor in the carboxylation of osteocalcin that is produced by osteoblasts. The effect of dietary intake of 45 μg MK-7 on the circulating levels of undercarboxylated osteocalcin and carboxylated osteocalcin was examined in healthy prepubertal children [13]. Supplementation with MK-7 for 8 weeks was found to increase circulating concentrations of MK-7 and enhance osteocalcin carboxylation in healthy children (n=55) [13]. The circulating concentrations of K1,, MK-4 and MK-7 in healthy women (n= 396) were also examined to ascertain whether each form of vitamin K is significantly associated with bone metabolism [14]. Circulating concentrations of MK-7 were found to be the highest and MK-4 was the lowest of the 3 vitamers in all age groups [14]. K1,and MK-7 were inversely correlated with undercarboxylated osteocalcin, but it was not observed that associations were not seen between nutritional basal concentration of MK-4 and under carboxylated osteocalcin [14]. Plasma K1 or MK-7 concentration, which is required to minimize the undercarboxylatedosteocalcin concentration, was shown to be the highest in the group aged over 70 years [14]. Circulating vitamin K concentrations in elderly people were needed to maintain at higher levels than those in young people to prevent bone loss and fracture [14].
Daily intake of about 100 μg of vitamin K1 is recommended for the maintenance of hemostasis in adults [15]. Vitamin K deficiency was seen in the hospitalized patient including malabsorption syndromes (especially owing to chlestatic liver disease), antibiotic therapy, and renal insufficiency [15]. Pregnant women and their newborns presented a special risk category, because of poor placental transport and low concentrations of vitamin K in breast milk [15]. The Food and Drug Administration mandated that adult parenteral preparations should provide a supplemental amount of 150 μg vitamin K1,per day [15]. This daily amount may be beneficial in preventing vitamin K deficiency [15].
Physiological roles of vitamin K2, are found in bone mineralization, arterial calcification, apoptosis, phagocytosis, growth control,chemotaxis and signal transduction. Especially, there is accumulating evidence for the roles of vitamin K in bone and vascular health. Vitamin K2, increased osteoblastic activity that enhances mineralization in vitro. Clinically, vitamin K2, maintained lumbar bone mineral density (BMD) and prevents osteoporotic fractures in patients with osteoporosis.
Anabolic effects of vitamin K on bone metabolism have been reported in several studies [15-20]. MK-4 increased the amount of osteocalcin and mineralization in cultured human osteoblasts [17]. MK-4 is essential for the γ-carboxylation of osteocalcin that are synthesized in osteoblasts of bone tissues [18]. Noncarboxylated osteocalcin cannot bind to hydroxyapatite in mineralized tissues [19]. MK-4 was also found to inhibit bone resorption and prevent bone loss in ovariectomized rats [20].
MK-7 contains very abundant in fermented soybean(natto),although it is only slight in other foods. Serum MK-7 concentration in women living in Tokyo, where fermented soybean is consumed, is about ten times higher than that of those living in Europe [21]. These differences may result from the intake ofnatto.Intake of MK-7 through diet may be beneficial effect in bone metabolism. This author found that MK-7 reveals an anabolic effect on bone calcification in rat femoral tissuesin vitro[22]. This was the first time finding that MK-7 reveals a direct anabolic effect on bone tissues. In addition, MK-7 was demonstrated to stimulate calcification in the femoral-metaphyseal tissues obtained from young and aged ratsin vitro[23]. Action of MK-7 on bone calcification revealed the same effect as compared with that of MK-4 [23]. MK-7 is partially converted to MK-4 in the body. Vitamin K2,may be important in maintaining bone healthy [24,25].
Osteocalcin, which is a bone matrix protein, is synthesized in osteoblasts with vitamin K dependent in the bone tissues. The fraction newly synthesized by osteoblasts is released into circulation. Therefore, the circulating levels of osteocalcin are considered sensitive markers of bone formation [26,27]. Among various types of osteocalcins, fully carboxylatedosteocalcin has a high affinity to calcium and/or hydroxyapataite and may play an important role in maintaining BMD and reducing the incidence of fractures through incremental bone formation [26,27].
Several studies in adults suggested a beneficial role for vitamin K in bone mineral metabolism and bone fracture prevention [14]. Poor vitamin K status may lead to production of undercarboxylated osteocalcin [28]. In the healthy adult population, osteocalcin was carboxylated to a variable extent, suggesting that the intake of dietary vitamin K is often insufficient for carboxylation of osteocalcin. Elevated undercarboxylated osteocalcin and fracture risk were occurred in postmenopausal women [14].
Patients with hip fracture showed lower vitamin K1, levels [25]. Lower levels of vitamin K1, and vitamin K2, were found in the serum obtained from elderly patients after a hip fracture [29]. A low vitamin K1, intake is one of the risk factors in hip fracture. Higher vitamin K1, levels than the current recommendation for coagulation parameters may be required for carboxylation of osteocalcin [30]. The fractions of osteocalcin with a high affinity to hydroxyapatite were increased following vitamin K2, administration in association with a reduction in urinary calcium excretion [31]. A daily vitamin K1, supplement of 80 μg was suitable to reaching a premenopausal carboxylatedosteocalcin/ total osteocalcin ratio [32]. Vitamin K deficiency may be responsible for reduced carboxylation of osteocalci. This may be one of the risk factors in low bone mass resulting in fragility fractures. Intake ofdietary vitamin K may ameliorate undercarboxylation of osteocalcin in the elderly with osteoporosis and is potential beneficial effect on bone.
MK-7, which was isolated fromnatto,has been shown to stimulate bone calcification in the femoral tissues obtained from normal young and aged ratsin vitro[22]. Culture with MK-7 (10-6or 10-5M) caused a significant increase in biochemical components (alkaline phosphatase activity, DNA and calcium contents) in the femoral-cortical and -trabecular tissues obtained from aged ratsin vitro[23]. Such an effect of MK-7 was also enhanced in the presence of genistein [23]. Anabolic effect of MK-7 in the bone tissues was based on newly synthesized protein componentsin vitro[23]. Moreover, MK-7 caused a significant increase in alkaline phosphatase activity, protein (including osteocalcin) and DNA contents in osteoblastic MC3T3-E1 cellsin vitro[33]. The effect of MK-7 in osteoblastic cells was completely depressed in the presence of protein inhibitor. Thus, MK-7 has been demonstrated to stimulate osteoblastic bone formation due to increasing protein synthesis [33].
The effects of MK-7 on various gene expressions, which are related to osteoblastic function, have been shown in osteoblastic MC3T3E1 cellsin vitro[34]. MK-7 did not have effects on the proliferation of osteoblastic cell cultured for 4 days [34]. However, cell proliferation was suppressed in osteoblastic cells cultured with MK-7 for 10 days [34]. MK-7 stimulated the gene expression of osteocalcin, osteoprotegerin, the receptor activator of the NFκB ligand (RANKL) and RANK in osteoblastic cells [34]. MK-7 may regulate gene expressions in osteoblastic MC3T3-E1 cells [34].
Osteoblast is differentiated from bone marrow mesenchymalstem cells. MK-7 enhanced vitamin D3-induced gene expression of osteocalcin in mesenchymal stem cells [35]. Among genes related to cell growth and differentiation, a specific effect of MK-7 was found in growth differentiation factor-10 and insulin-like growth factor 1, and the latter being also involved in the induction of vascular endothelial growth factors [35].
Moreover, MK-7 has been shown to be a transcription regulator of bone-specific genes that act through steroid and xenobiotic receptors (SXRs), which stimulates transcription process of osteoblastic markers [36].
MK-7 has been demonstrated to have suppressive effects on oteoclastic bone resoptioninvitro,[37]. MK-7 was found to reveal suppressive effects on the decrease in calcium content in rat femoral tissues, which are induced after culture with bone-resorbing factors including parathyroid hormone (PTH) and prostaglandin E2 (PGE2), demonstrating its suppressive effects on bone resorptionin vitro[37]. Suppressive effects of MK-7 on bone resorption were partly involved in inhibition for PTH- or PGE2-stimulated increase in lactic acid, which is produced by medium glucose consumption in the bone tissuesinvitro[37].
Osteoclastic cells are formed from bone marrow cells in the presence of bone-resorbing factors (PTH, PGE2 and other) [37]. MK-7 (10-8– 10-5M) was found to suppress PTH- or PGE2-induced increase in osteoclast-like cell formation in mouse bone marrow cell culturein vitro[37]. MK-7 revealed more potent-suppressive effects at later stage of the differentiation of bone marrow cells. MK-7 suppressed osteoclastogenesis after culture with phorbol 12-myristate 13-acetate (PMA), an activator of protein kinase C [37]. MK-7 may suppress activation of protein kinase C signaling [37]. However, MK-7 did not inhibit cyclic AMP-dependent signaling-stimulated osteoclastogenesisin bone marrow cell culturein vitro[37].
The effect of MK-7 on mature osteoclasts isolated from rat femoral tissues has also been examined [37]. MK-7 was found to decrease in the number of mature osteoclastsin vitro,inducing cell apoptosis [37]. This effect was partly mediated through suppressive action of MK-7 for the pathways of Ca2+- and cyclic AMP-dependent signaling [37].
MK-7 stimulated osteoblastic bone formation and suppresses osteoclastic bone resorption. MK-7 increased protein synthesis including osteocalcin and activates γ-carboxylase in osteoblastic cells. Moreover, MK-7 was found to suppress activation of NF-κB signalling in both osteoblastogenesis and osteoclastogenesis. Activation of NF- κB signal transduction pathway is essential for osteoclast formation. Also, NF-κB signaling potently antagonizes osteoblast differentiation in osteoblastic cells [38]. Cytokine-induced NF-κB activation inhibits differentiation of pre-osteoblastic cells and stimulates the formation of osteoclasts [38]. MK-7 was found to restore depression by TNF-α of Smadsignaling induced by either TGF-β or BMP-2 [38]. MK-7 further antagonized receptor activator of NF-κB (RANK) ligand (RANKL)-induced NF-κB activation in osteoclast precursors [38]. MK-7 stimulated IκB mRNA expression [38]. Thus MK-7 down-regulated basal and cytokine-induced NF-κBactivation, and this action was a γ-carboxylation-independent manner [38]. These findings provide a novel mechanism of vitamin K2, which stimulates osteblastic bone formation and suppresses osteoclastic bone resorption, as shown in Figure 2.
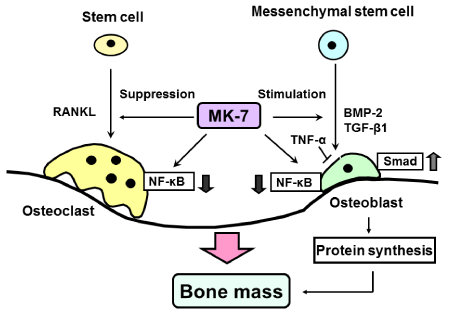
Cellular mechanism by which menaquinone-7 (MK-7) stimulates osteoblastic bone formation and suppresses osteoclastic bone resorption. MK-7 suppresses NF-κB signaling pathways, which are activated by stimulation of TNF-α or RANKL, in osteoblasts and osteoclasts. In addition, MK-7 stimulates protein synthesis (including osteocalcin and other protein molecules) in osteoblastic cells, and it may regulate the gene expression of various proteins, which are related to cellular functions in osteblastic and osteoclastic cells.
MK-7 increases bone mass due to stimulating osteoblastic bone formation and inhibiting osteoclastic bone resorption, suggesting that the vitamin K2 has a preventive effect on osteoporosis. Preventive effects of dietary MK-7 on bone loss have been shown using ovariectomized (OVX) rats, animal models for postmenopausal osteoporosis [39]. MK-7 was found to have preventive effects on OVX-induced decrease in the femoral dry weight and calcium content [39]. In separate experiments, OVX rats were given experimental diets containing fermented soybeans (natto;including MK-7, 9.4 μg/100 g diet) with or without added MK-7 (37.6 μg/100 g diet) for 77 days [39]. Dietary MK-7 prevented OVX-induced decreases in the femoral dry weight and femoral calcium content [39]. Thus dietary MK-7 was demonstrated to reveal preventive effects on bone loss in menopausal osteoporosis model animalsin vivo.
The effect of prolonged intake of dietary MK-7 on bone loss in OVX rats was also found [40]. Dietary MK-7 (9.4 μg/100 g diet) with or without supplemental MK-7 (containing 14.1 or 18.8 μg/100 g diet) was given to OVX rats for 150 days [40]. Feeding produced an elevation of serum concentration of MK-7 in OVX rats [40]. Feeding of MK-7 (18.8 μg/100 g diet) prevented decreases in serum γ-carboxylatedosteocalcinconcentration, femoral dry weight, femoral calcium content, and mineral density that are induced by OVX [40]. Prolonged intake of dietary MK-7 prevented OVX-induced bone loss. Dietary MK-7 may be useful in the prevention of osteoporosis.
Combination of nutritional factors may have additive or synergistic effects in the prevention of bone loss with increasing age. Interestingly, the anabolic effect of MK-7 on bone components was found to synergistically enhance with combination of zinc in rats invivo [41]. Supplemental intake with combination of MK-7 and zinc may be potential in the prevention and treatment of osteoporosis with increasing age.
Fermented soybeans(natto),which contain a large amount ofMK-7, may help in the prevention of osteoporosis. Epidemiologic data suggested that intake ofnattomay play a preventive role for osteoporosis [21]. Moreover, it was demonstrated that prolonged intake ofnattobrings an increase in MK-7 and a corresponding elevation of γ-carboxylatedosteocalcin in the serum of normal human individuals (forty-eight volunteers; 45 men and 3 women) [42,43]. Serum MK-7 was not found in normal individuals without dietarynattointake. Increases in serum MK-7 and γ-carboxylated osteocalcin concentrations were observed for 14 days after the start of dietarynattointake containing MK-7 1295 or 1730 μg/100 g diet [43]. Appropriate amount of dietary MK-7 may bring benefic effects on bone mineralization in human individuals. Supplemental intake of MK-7 may be a useful tool in the prevention and treatment of bone loss with aging.
The possibility of an association between habitualnattointake and bone mineral density (BMD) and BMD change was studied in healthy Japanese women who participated in a large representative cohort study (Japanese Population-based Osteoporosis Study: JPOS study) [44]. BMD was measured at the spine, hip and forearm in 944 women (20–79 years old) at baseline and at a follow-up conducted 3 years later [44]. The total hip BMD was found to elevate with increasing of nattointake in the postmenopausal women [44]. In addition, there were observed significant positive associations between nattointake and the rates of changes in BMD at the femoral and at the distal third of the radius in the postmenopausal women [44]. Dietarynattointake was suggested to prevent postmenopausal bone loss through the effects of menaquinone 7 that are more abundantin nattothan in other soybean products.
Supplementation with 3-year high-dose vitamin K1(phylloquinone) and K2 (MK-4) has been shown to improve bone health after menopause [45]. Intake of MK-7 improved vitamin K status and to decrease the age-related decline in BMD at the lumbar spine and femoral neck, when healthy postmenopausal women (n=244) were received for 3 years placebo or MK-7 (180 μg MK-7/day) capsules [45]. Intake of MK-7 was also found to increase bone strength and prevent the loss in vertebral height of the lower thoracic region at the mid-site of the vertebrae [45]. MK-7 supplements may prevent bone loss in postmenopausal women.
Moreover, the effect of dairy products enriched with calcium, vitamin D3, and vitamin K1or MK-7 on parameters of bone metabolism was shown in postmenopausal women following a 12-month intervention [46]. Postmenopausal women received fortified dairy products providing either daily 800 mg of calcium and 10 μg of vitamin D3 (CaD) or daily 100 μg of either vitamin K1 (CaDK1) or MK-7 (CaDK2) [46]. Increase in the serum 25-hydroxyvitamin D3 levels was observed in all intervention groups [46]. Decrease in serum undercarboxylated osteocalcin to osteocalcin ratio and urine deoxypyridinoline levels in both the CaDK1 and CaDK2 groups was also seen as compared tothe CaD and control groups [46]. Moreover, significant increases in BMD in total-body and lumbar spine were observed only for CaDK1 and CaDK2 [46]. This study showed more favorable changes in bone metabolism and bone mass with supplementation of vitamin K [46]. Thus supplemental MK-7 may have benefic effects in the prevention and treatment of bone loss in postmenopausal women.
Vitamin K2, which was discovered as blood coagulation factor, may play multifunctional roles in the living body. Vitamin K2 plays an essential role as co-factor of γ-carboxylase that converted glutamate residues of osteocalcin into γ-carboxyglutamate, suggesting its role in bone metabolism. In this review, the role of MK-7 in bone homeostasis has been focused. MK-7 stimulates osteoblastic bone formation and inhibits osteoclastic bone resorption. This action of MK-7 has been demonstrated to mediate through the pathways of Ca2+- and cyclic AMP-dependent signaling and the antagonizing cytokine-induced NF- κB activation in a γ-carboxylation-independent manner. Moreover, MK-7 regulates a transcription of bone-specific genes that act through steroid and xenobiotic receptors to promote the expression of osteoblastic marker proteins, and it also stimulates the synthesis of various proteins including osteocalcin in bone cells. Further studies remains to be elucidated molecular and cellular mechanisms of MK-7. Several studies have demonstrated that dietary intake of MK-7 has benefit effects to prevent bone loss and bone fracture in normal postmenopausal women. MK-7 may be useful in the prevention and amelioration of osteoporosis. Moreover, MK-7 may suppress boneloss in various disease states including inflammation, obesity, diabetes, and cancer bone metastastasis. Drugs, which are used clinically in the treatment of osteoporosis, are mainly based on the action of osteoclastic bone resorption. Clinical compounds that stimulate bone formation are under development. MK-7 will be expected as a potentialosteogenic factor in the treatment of osteoporosis.
2021 Copyright OAT. All rights reserv