Abstract
Background: The risks and benefits of different duration of dual antiplatelet therapy (DAPT) after drug-eluting stent (DES) implantation remains a matter of debate.
Objectives: To systematically review the risks and benefits of 12-month vs. >12-month dual antiplatelet therapy (DAPT) after drug-eluting stent (DES) implantation.
Methods: Randomized controlled trials about duration of DAPT after DES implantation was searched through the PubMed, Cochrane Library, EMbase and Web of Science. No limitations regarding the language of publications.
Results: Six randomized controlled studies involving 25054 patients were included. The results of meta-analysis showed: 12-month DAPT was associated with a lower risk of all-cause mortality (FEM; RR=0.76; 95%CI, 0.63 to 0.92; Z=2.78; P=0.006) and a higher risk of stent thrombosis(FEM; RR=2.48; 95%CI, 1.72 to 3.56; Z=4.88; P<0.00001), myocardial infarction ( FEM; RR=1.71; 95%CI, 1.44 to 2.04; Z=6.02; P<0.00001). The rates of stroke, TVR, major bleeding and cardiac mortality had no significant difference between 12-month DAPT and >12-month DAPT.
Conclusion: 12-month dual antiplatelet therapy after drug-eluting stent implantation was associated with a lower risk of all-cause mortality and a higher risk of myocardial infarction and stent thrombosis. The rates of stroke, TVR, major bleeding and cardiac mortality had no significant difference between 12-month DAPT and >12-month DAPT.
Keywords
Drug-eluting stent; Dual antiplatelet therapy; Meta-analysis
Introduction
The American College of Cardiology and American Heart Association(ACC/AHA) guidelines recommend that dual-antiplatelet therapy(DAPT) be administered for at least 12 months after drug-eluting stent(DES) implantation [1], while the European Society of Cardiology and European Association for Cardio-Thoracic Surgery(ESC/EACTS) recommend 6-12 months [2]. Therefore, the optimal duration of dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor after drug-eluting stent implantation remains a matter of debate. What would be the safest and shortest DAPA duration after DES implantation?
Methods
In this meta-analysis, S-DAPT was defined as 12-month DAPT after DES implantation; L-DAPT was defined as >12-month (24 months or 30 months or 36 months or 48 months) DAPT after DES implantation. The outcomes included all-cause mortality, cardiac mortality, MI, repeat emergency TVR, stroke, stent thrombosis, major bleeding. MI included Q-wave and non-Q-wave MI. Repeat emergency target vessel revascularization (TVR) was defined as emergency repeat coronary revascularization of any segment of the treated coronary artery within 12 months of stenting. Stroke was defined as acute new neurological deficit ending in death or lasting longer than 24 h, diagnosed as stroke by a physician. The TIMI classification was used to define major bleeding.
We systematically searched MEDLINE, the Cochrane Library, EMbase and Web of Science (from inception up to November 16, 2016) to identify relevant randomized controlled trials (RCTs) by using the search terms: drug-eluting stent AND dual antiplatelet therapy AND Random. The reference lists of the initially retrieved articles were also reviewed. No limitations regarding the country, time or language of publications.
Data Extraction
Two investigator dependently reviewed the full text of the retrieved articles and reported the results in a structured dataset. Disparities between investigators regarding the inclusion of each trial were resolved by consensus by a third independent investigator. The data’s included first author, year, country of publication, study design, S-DAPT and L-DAPT duration, maximum length of follow-up, sample size, outcome measures and endpoints of interest. The assessment of the methodological quality of the included RCTs was followed the recommendations exemplified in the Cochrane handbook for systematic reviews of interventions and summarized in a domain based evaluation of the following components: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias [3].
Statistical Analysis
We used Review Manager 5.3 to conduct the statistical analysis. The between-study heterogeneity was assessed by the chi-squared test and its extent was quantified by the I2 statistic (I2 values of 25%, 50%, and 75% were considered to represent low, moderate and severe statistical inconsistency [4]. Continuous outcomes were analyzed using mean differences (MD) and 95% confidence intervals (CIs). Risk ratio (RR) and 95% CI were calculated by implementing the Mantel-Haenszel fixed effect model (FE) and the Mantel-Haenszel random effect model (RE). A p<0.05 was thought to indicate statistical significance in this meta-analysis.
Results
The study analysis flow diagram is shown in Figure 1. Of the 1039 citations found in MEDLINE, the Cochrane Library, EMbase and Web of Science, 6 trials met the inclusion criteria and the majority was excluded for reasons presented in Figure 1. Thus, six RCTs were included in the final meta-analysis [5-10].
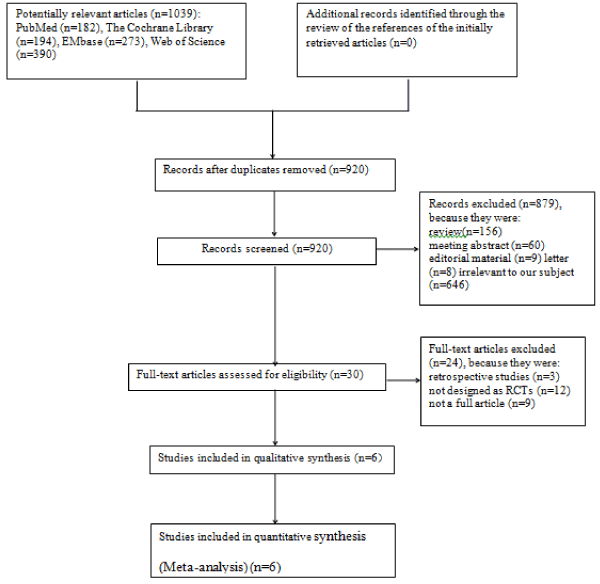
Figure 1: Flow diagram of the literature search and selection process of the studies.
In Table 1, we list the main characteristics of the included studies that meet our eligibility criteria. All studies [5-10] were published from 2010 to 2016. Their individual sample size ranged from1259 to 9961 patients. All of the six trials were multi-center studies. Two RCTs evaluated a12-month versus 30-month DAPT regimen. One RCT evaluated a 12-month versus a 24-month DAPT regimen. One RCT evaluated a 12-month versus a 36-month DAPT regimen. One RCT evaluated a 12-month versus a 48-month DAPT regimen. One RCT evaluated a 12-month versus an 18-30-month DAPT regimen. Data regarding the S-DAPT vs. L-DAPT are presented in Table 2. Data on the quality of the included studies are presented in Table 3.
Table 1: Main characteristics of the studies included in the meta-analysis: S-DAPT vs. L-DAPT.
Source/Year [Reference] |
Country |
Study Design |
Definition of short and long duration |
Total number in each group |
Follow-up Duration |
Collet/2014 [6] |
France |
P, PG, OL, MC |
12 vs. 18-30 months |
624 vs. 635 |
30 months |
Park/2010 [10] |
South Korea |
P, PG, OL, MC |
12 vs. 24 months |
1344 vs. 1357 |
24 months |
Hermiller/2016 [7] |
International |
P, PG, DB, MC |
12 vs. 30 months |
2358 vs. 2345 |
30 months |
Mauri/2014 [9] |
International |
P, PG, SB, MC |
12 vs. 30 months |
4941 vs. 5020 |
30 months |
Lee/2014 [8] |
Korea |
P, PG, OL, MC |
12 vs. 36 months |
2514 vs. 2531 |
24 months |
Helft/2015 [5] |
International |
P, PG, OL, MC |
12 vs. 48 months |
690 vs. 695 |
48 months |
P, prospective; PG, parallel group; OL, open label; SB, single-blind; DB, double-blind; MC, multi-center; SC, single-center;
Table 2: Outcome data of the studies included in the meta-analysis: 12-month DAPT vs. >12-month DAPT
Source/Year (Reference) |
Collet/2014 [6] |
Park/2010 [10] |
Hermiller/2016 [7] |
Mauri/2014 [9] |
Lee/2014 [8] |
Helft/2015 [5] |
all-cause mortality |
8 (0.9%) vs. 7 (0.8%) |
5 (0.7%) vs. 10 (1.5%) |
26 (1.1%) vs. 49 (2.2%) |
13 (1.0%) vs. 20 (1.5%) |
6 (0.6%) vs. 11 (1.3%) |
32 (1.4%) vs. 46 (2.0%) |
cardiac mortality |
5 (0.5%) vs. 3 (0.3%) |
3 (0.4%) vs. 5 (0.7%) |
18 (0.8%) vs. 23 (1.0%) |
NA |
4 (0.4%) vs. 7 (0.7%) |
19 (0.8%) vs. 28 (1.2%) |
myocardial infarction |
6 (0.7%) vs. 4 (0.4%) |
1 (0.1%) vs. 0 |
72 (3.2%) vs. 48 (2.1%) |
7 (0.5%) vs. 10 (0.7%) |
2 (0.2%) vs. 4 (0.4%) |
27 (1.2%) vs. 19 (0.8%) |
stroke |
0 vs. 4 (0.4%) |
6 (0.9%) vs. 3 (0.4%) |
15 (0.7%) vs. 13 (0.6%) |
4 (0.3%) vs. 9 (0.7%) |
5 (0.5%) vs. 6 (0.7%) |
21 (0.9%) vs. 21 (0.9%) |
TVR |
5 (0.5%) vs. 2 (0.2%) |
29 (4.4%) vs. 21 (3.1%) |
NA |
26 (1.9%) vs. 36 (2.7%) |
27 (3.7%) vs. 27 (3.7%) |
65 (2.8%) vs. 81 (3.5%) |
Stent thrombosis |
3 (0.3%) vs. 0 |
2 (0.3%) vs. 2 (0.3%) |
16 (0.7%) vs. 6 (0.3%) |
4 (0.3%) vs. 5 (0.4%) |
2 (0.2%) vs. 3 (0.3%) |
11 (0.5%) vs. 7 (0.3%) |
Major bleeding |
0 vs. 3 (0.3%) |
5 (0.7%) vs. 7 (1.0%) |
NA |
1 (0.1%) vs. 3 (0.2%) |
2 (0.2%) vs. 6 (0.6%) |
24 (1.1%) vs. 34 (1.4%) |
Table 3: Assessment of the methodological aspects of the included studies.
Source/Year (Reference) |
Bias Component (Sequence generation and allocation concealment) |
Blinding |
Incomplete outcome data |
Selective outcome reporting |
Other sources of bias |
Quality score |
Collet/2014 [6] |
Using a computer-generated randomization sequence (1:1; stratified by center) |
No |
99 patients withdrew consent. |
Extensive reporting |
did not enroll patients at very high thrombotic burden |
High |
Park/2010 [10] |
According to a pre-established, computer-generated randomization scheme, with stratification on the basis of site and type of drug (sirolimus, paclitaxel, or zotarolimus) in the drug eluting stent. |
No |
No |
Extensive reporting |
the rate of the primary
end point was lower than expected, |
High |
Hermiller/2016 [7] |
Randomization was stratified by DES/BMS, hospital site, subject complexity, and thienopyridine drug type. |
Double-blind |
129 patients withdrew consent. |
Extensive reporting |
tests of interaction on randomized treatment
effect between stent groups remain underpowered. |
High |
Mauri/2014 [9] |
Randomization was conducted in a 1:1 ratio. A computer-generated randomization schedule stratified patients according to the type of stent they had received |
Single-blind |
Almost 1 every 10 screened patients was excluded from the study |
Extensive reporting |
only patients who were adherent to therapy and who did not have a major adverse cardiovascular or cerebrovascular event,
stent thrombosis, or moderate or severe bleeding
in the first year underwent randomization, a study design that may have selected for patients who were at lower risk for late adverse events. |
High |
Lee/2014 [8] |
The treatment group assignments were made according to a pre-established, computer-generated randomization scheme that involved stratification on the basis of the site and the type of drug in the drug-eluting stent. |
No |
Almost 1 every 10 screened patients was excluded from the study |
Extensive reporting |
the study was an open label
trial without a placebo control. |
High |
Helft/2015 [5] |
Patients were randomly assigned to either a short group or long group without further reported details |
No |
4 patients withdrew consent. |
Extensive reporting |
only powered to detect major differences in ischemic and bleeding events |
High |
All-cause mortality
Six RCTs (5-10) in this meta-analysis provided data on the all-cause mortality. No Significant heterogeneity was detected between these studies (Chi2=8.44, df=5; P=0.13; I2=41%). The combined estimate for the all-cause mortality based on the fixed-effects model showed statistically significant difference between the S-DAPT group and the L-DAPT group(24595 patients; FEM; RR=0.76; 95%CI, 0.63 to 0.92; Z=2.78; P=0.006) (Figure 2).
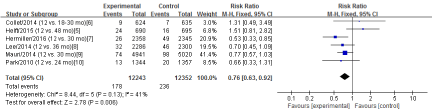
Figure 2: The all-cause mortality: forest plot showing the comparison of S-DAPT vs. L-DAPT. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the risk ratio (RR) for all studies.
Cardiac Mortality
Four RCTs [5,7-9] in this meta-analysis provided data on the cardiac mortality. No significant heterogeneity was detected between these studies (Chi2=2.93, df=3; P=0.40; I2=0%). The combined estimate for the cardiac mortality based on the fixed-effects model showed no statistically significant difference between the S-DAPT group and the L-DAPT group (20604 patients; FEM; RR=0.93; 95%CI, 0.71 to 1.23; Z=0.50; P=0.61) (Figure 3).
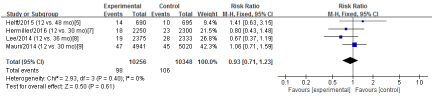
Figure 3: The cardiac mortality: forest plot showing the comparison of S-DAPT vs. L-DAPT. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the risk ratio (RR) for all studies.
Myocardial Infarction
Six RCTs [5-10] in this meta-analysis provided data on the rates of myocardial infarction. No Significant heterogeneity was detected between these studies (Chi2=7.23, df=5; P=0.20; I2=31%). The combined estimate for the rates of myocardial infarction based on the fixed-effects model showed statistically significant difference between the S-DAPT group and the L-DAPT group(24467 patients; FEM; RR=1.71; 95%CI, 1.44 to 2.04; Z=6.02; P<0.00001) (Figure 4).
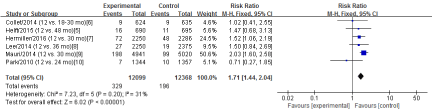
Figure 4: The rates of myocardial infarction: forest plot showing the comparison of S-DAPT vs. L-DAPT. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the risk ratio (RR) for all studies.
Stroke
Six RCTs [5-10] in this meta-analysis provided data on the rates of stroke. No significant heterogeneity was detected between these studies (Chi2=3.11, df=5; P=0.68; I2=0%). The combined estimate for the rates of stroke based on the fixed-effects model showed no statistically significant difference between the S-DAPT group and the L-DAPT group (24282 patients; FEM; RR=1.04; 95%CI, 0.78 to 1.39; Z=0.29; P=0.77) (Figure 5).
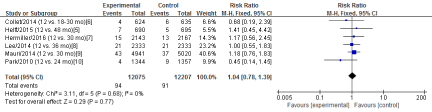
Figure 5: The rates of stroke: forest plot showing the comparison of S-DAPT vs. L-DAPT. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the risk ratio (RR) for all studies.
Stent Thrombosis
Six RCTs [5-10] in this meta-analysis provided data on the stent thrombosis. No Significant heterogeneity was detected between these studies (Chi2=8.68, df=5; P=0.12; I2=42%). The combined estimate for the rates of stent thrombosis based on the fixed-effects model showed statistically significant difference between the S-DAPT group and the L-DAPT group(24125 patients; FEM; RR=2.48; 95%CI, 1.72 to 3.56; Z=4.88; P<0.00001) (Figure 6).
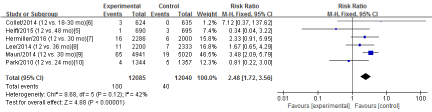
Figure 6:
TVR
Four RCTs [5-6,8,10] in this meta-analysis provided data on the rates of TVR. No significant heterogeneity was detected between these studies (Chi2=1.41, df=3; P=0.70; I2=0%). The combined estimate for the rates of TVR based on the fixed-effects model showed no statistically significant difference between the S-DAPT group and the L-DAPT group (9980 patients; FEM; RR=0.85; 95%CI, 0.68 to 1.06; Z=1.45; P=0.15) (Figure 7).
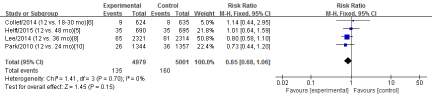
Figure 7: The rates of TVR: forest plot showing the comparison of S-DAPT vs. L-DAPT. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the risk ratio (RR) for all studies.
Major Bleeding
Four RCTs [5-6,8,10] in this meta-analysis provided data on the rates of major bleeding. No Significant heterogeneity was detected between these studies (Chi2=3.07, df=3; P=0.38; I2=2%). The combined estimate for the rates of major bleeding based on the fixed-effects model showed no statistically significant difference between the S-DAPT group and the L-DAPT group(9956 patients; FEM; RR=0.68; 95%CI, 0.43 to 1.07; Z=1.67; P=0.09) (Figure 8).
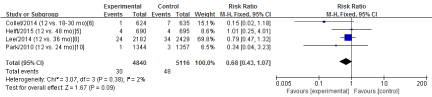
Figure 8: The rates of major bleeding: forest plot showing the comparison of S-DAPT vs. L-DAPT. The size of each square represents the proportion of information provided by each study. The vertical line depicts the point of “no difference” between the two groups, and the horizontal lines correspond to the 95% confidence intervals (CIs). Diamonds represent the risk ratio (RR) for all studies.
Publication bias
Six studies [5-10] were included in this meta-analysis. Assessment of publication bias using a funnel plot was presented in Figure 9.
Figure 9: Funnel plot.
Discussion
This meta-analysis included six randomized controlled studies involving 25054 patients. The main results of this meta-analysis are as follow:
1) 12-month DAPT was associated with a lower risk of all-cause mortality.
2) 12-month DAPT was associated with a higher risk of myocardial infarction and stent thrombosis.
3) The rates of stroke, TVR, major bleeding and the cardiac mortality had no significant difference between the 12-month DAPT and >12-month DAPT.
Optimal DAPT duration is critical for balancing the risk of ischemic and bleeding complications after DES implantation. Elmariah, et al. [11] founded that patients’ mortality was no significant difference between short term of DAPT and long term of DAPT after DES implantation. Giustino, et al. [12] confirmed that all-cause mortality was numerically higher with long term of DAPT without reaching statistical significance. However, in this meta-analysis, we found that the short term of DAPT was associated with a lower risk of all-cause mortality (FEM; RR=0.76; 95%CI, 0.63 to 0.92; Z=2.78; P=0.006). While the cardiac mortality between the short DAPT and long DAPT had no significant difference (FEM; RR=0.93; 95%CI, 0.71 to 1.23; Z=0.50; P=0.61).
Recently, trials demonstrated that Prolonged DAPT reduced stent-related and non-stent-related adverse ischemic events following PCI [13]. American guidelines recommend at least 12 months of DAPT after DES implantation in order to reduce the rates of late and very late stent thrombosis. In this meta-analysis ,results showed that short term of DAPT (12 months) was associated with a higher risk of stent thrombosis(FEM; RR=2.48; 95%CI, 1.72 to 3.56; Z=4.88; P<0.00001), as well as myocardial infarction (FEM; RR=1.71; 95%CI, 1.44 to 2.04; Z=6.02; P<0.00001).
The definition of major bleeding complications varies widely across clinical studies. In this meta-analysis, we adopted the TIMI major bleeding scale. The results showed that the major bleeding between 12-month DAPT and >12-month DAPT after drug-eluting stent implantation had no significant difference (FEM; RR=0.68; 95%CI, 0.43 to 1.07; Z=1.67; P=0.09).
The results of this meta-analysis confirm and extend the previous reports [14-21]. However, this meta-analysis has several limitations. First, not all included studies provided data on all outcomes. Second, four trials included in the meta-analysis were open label, potentially introducing performance bias. Third, all of the patients included in the meat-analysis were treated with clopidogrel as adjunctive therapy to aspirin. It remains unclear whether results would have differed with the other kind of P2Y12 inhibitor, especially in patients with acute coronary syndrome.
Conclusion
In conclusion, 12-month dual antiplatelet therapy after drug-eluting stent implantation was associated with a lower risk of all-cause mortality and a higher risk of myocardial infarction and stent thrombosis. The rates of stroke, TVR, major bleeding and cardiac mortality had no significant difference between 12-month DAPT and >12-month DAPT. Further research is required to determine the duration of DAPT after DES implantation in patients with ACS.
References
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, et al. (2011) ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124: e574-651. [CrossRef]
- Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, et al. (2014) ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 46: 517-592. [CrossRef]
- Higgins JP, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0).
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560. [CrossRef]
- Helft G, Steg PG, Le Feuvre C, Georges JL, Carrie D, et al. (2016) Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 37: 365-374. [CrossRef]
- Collet JP, Silvain J, Barthélémy O, Rangé G, Cayla G, et al. (2014) Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 384: 1577-1585. [CrossRef]
- Hermiller JB, Krucoff MW, Kereiakes DJ, Windecker S, Steg PG, et al. (2016) Benefits and Risks of Extended Dual Antiplatelet Therapy After Everolimus-Eluting Stents. JACC Cardiovasc Interv 9: 138-147. [CrossRef]
- Lee CW, Ahn JM, Park DW, Kang SJ, Lee SW, et al. (2014) Optimal Duration of Dual antiplatelet Therapy After Drug-Eluting Stent Implantation: A Randomized, Controlled Trial. Circulation 129: 304-312. [CrossRef]
- Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, et al. (2014) Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N Engl J Med 371: 2155-2166. [CrossRef]
- Park SJ, Park DW, Kim YH, Kang SJ, Lee SW, et al. (2010) Duration of Dual Antiplatelet Therapy after Implantation of Drug-Eluting Stents. N Engl J Med 362: 1374-1382. [CrossRef]
- Elmariah S, Mauri L, Doros G, Galper BZ, O'Neill KE, et al. (2015) Extended duration dual antiplatelet therapy and mortality: a systematic review and meta-analysis. Lancet 385: 792-798. [CrossRef]
- Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, et al. (2015) Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 65: 1298-1310. [CrossRef]
- Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, et al. (2014) Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 371: 2155-2166. [CrossRef]
- Valgimigli M, Park SJ, Kim HS, Park KW, Park DW, et al. (2013) Benefits and risks of long-term duration of dual antiplatelet therapy after drug-eluting stenting: a meta-analysis of randomized trials. Int J Cardiol 168: 2579-2587. [CrossRef]
- Cassese S, Byrne RA, Tada T, King LA, Kastrati A (2012) Clinical impact of extended dual antiplatelet therapy after percutaneous coronary interventions in the drug-eluting stent era: a meta-analysis of randomized trials. Eur Heart J 33: 3078-3087. [CrossRef]
- El-Hayek G, Messerli F, Bangalore S, Hong MK, Herzog E, et al. (2014) Meta-analysis of randomized clinical trials comparing short-term versus long term dual antiplatelet therapy following drug-eluting stents. Am J Cardiol 114: 236-242. [CrossRef]
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, et al. (2001) Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345: 494-502. [CrossRef]
- I-LOVE-IT 2 Trial (2016) Circul Cardiovas Interv 9: B38.
- Baber U, Mehran R, Sharma SK, Brar S, Yu J, et al. (2011) Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol 58: 1569-1577. [CrossRef]
- Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, et al. (2012) Biodegradable polymer drug-eluting stnets reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 33: 1214-1222. [CrossRef]
- Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, et al. (2007) Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115: 2435-2441. [CrossRef]









