Purpose: In this study we investigated 3D changes in craniofacial architecture, to test the hypothesis that the upper airway can be non-surgically improved in adults using biomimetic oral appliance therapy (BOAT).
Methods: After obtaining informed consent, we undertook 3D cone-beam computerized tomographic (CBCT) scans of 17 consecutive Korean adults who had been diagnosed with midfacial hypoplasia prior to BOAT. Post-treatment: follow-up 3D CBCT scans were undertaken with no device in the patient’s mouth; 3D craniofacial reconstruction was undertaken, including the upper airway (nasal cavity to retroglossal region), and pre- and post-treatment parameters were calculated; and the findings were subjected to bivariate and multivariate tests.
Results: The mean treatment time with BOAT was 16.6 months. Comparing mean pre- and post-treatment parameters: transpalatal bone width increased (p < 0.001); nasal airway volume increased (p < 0.01); inferior nasal concha distance from nasal septum remained relatively unchanged; area of posterior nasal apertures and soft palate length remained unchanged; antero-posterior retropalatal airway distance increased (p < 0.01); medio-lateral retropalatal distance remained unchanged (p > 0.05); retropalatal area increased (p < 0.01); sagittal retroglossal airway distance increased (p < 0.01); medio-lateral retroglossal width remained unchanged, while the retroglossal area increased (p < 0.05) with no device in the patient’s mouth. For the upper airway, the multivariate tests also showed a significant treatment effect (p < 0.05).
Conclusion: These findings corroborate the notion that craniofacial architecture and upper airway morphology can be non-surgically enhanced in non-growing adults.
upper airway, palatal expansion, obstructive sleep apnea, biomimetic oral appliance
Morphological determinants and craniofacial phenotypes are associated with obstructive sleep apnea (OSA) in both children and adults. A study on adults [1] reported that, using 2D cephalometric analysis, mandibular retrognathia was a feature of patients diagnosed with OSA compared to a control group. A similar study in adults also using cephalometry [2] found that mandibular retrognathia was associated with a narrowing of the upper airway in the sagittal plane. That these changes in mandibular form impact the upper airway is further supported by the finding that an increased thyromental angle, formed in part by the mandible, is associated with a greater likelihood of OSA in both Asian and Caucasian patients [3]. Indeed, in a 3D study, Banabilh et al. [4] confirmed finding a submandibular pannus in obese Asian patients testing positive for OSA. Wysocki et al. [5] concluded that the severity of OSA is associated with an increased neck circumference in males, a finding confirmed by Yilmaz et al. [6] who also found both the thyromental distance and neck circumference are increased in patients diagnosed with OSA. Therefore, changes in external craniofacial features are observable in OSA phenotypes.
Several techniques have also been deployed to investigate the upper airway phenotype of both pediatric and adult patients with OSA. In an early study, Rivlin et al. [7] used a combination of acoustic echography and cephalographic analysis to reveal that patients with OSA have a smaller cross-sectional pharyngeal area and a smaller or retrognathic mandible, which correlates with an increased apnea-hypopnea index (AHI) in patients with OSA. That these features represent an anatomic predisposition to upper airway occlusion was corroborated by Singh and Olmos [8], who used acoustic pharyngometry to demonstrate a technique for the prevention of upper airway collapse in adults. Krasny et al. [9] used computed tomography (CT) scans to investigate upper airway morphology in patients with OSA. They reported that the retropalatal distance (between the hard palate and posterior pharyngeal wall in the horizontal plane) as well as the distance between the soft palate and posterior pharyngeal wall, represent sites of pharyngeal obstruction regardless of obesity. More recently, Xu et al. [10] assessed craniofacial and upper airway structures in Asian-Chinese and European-Icelandic patients with OSA using 3D magnetic resonance imaging (MRI). They found that the Asians showed a smaller retropalatal airway size inter alia on MRI scans compared to the Europeans, which indicates the need for targeted therapies in cases of OSA as opposed to generic or non-customized approaches. In view of this requirement, the aim of this investigation is to localize and quantify changes in 3D craniofacial and upper airway architecture following biomimetic oral appliance therapy (BOAT) in Korean adults with midfacial hypoplasia to test the hypothesis that the upper airway can be non-surgically improved in adults and determine the utility of this novel approach on upper airway plasticity.
After obtaining informed consent, 14 consecutive patients were recruited from an orthodontic or dental office in South Korea for this investigation. The rights of the subjects were protected by following the 1964 Declaration of Helsinki and its later amendments. Inclusion criteria were: adults aged > 16yrs diagnosed with clinical midfacial hypoplasia (such as: narrow/high vaulted hard palate; crowding of the upper and/or lower teeth; anterior/posterior and/or unilateral/bilateral crossbite); no history of hospitalization for craniofacial trauma or surgery; no congenital craniofacial anomalies; a dentate upper arch, and good compliance. The exclusion criteria included: age < 16yrs; poor oral hygiene; active periodontal disease; systemic bisphosphonate therapy, and a lack of compliance. After careful history-taking and craniofacial examination, we undertook 3D scans using a CBCT machine (HDXwill, Dentri-s, Seoul, South Korea). Strict positioning protocols were used, and a 20 second scan was performed using a wide (13 cm) field of view. The scans were taken with patients in an upright position with the Frankfort plane parallel to the ground. All subjects were asked to maintain light centric occlusion, breathe normally and not to swallow while the scan was being taken. Next, a neuromuscular bite registration, which relaxes the muscles of mastication in order to achieve the physiologic rest position of the mandible [11], was obtained in the upright-sitting position with corrected jaw posture in the vertical axis for each subject. Upper and lower polyvinylsiloxane impressions were also obtained. Subsequently, the upper study model was mounted using the hamular notch-incisive papilla (HIP) plane method on a Stratos articulator (Ivoclar-Vivadent, Amherst, USA), and the lower model was mounted relative to the upper model, using the bite registration captured.
Following a diagnosis of midfacial hypoplasia, a biomimetic upper appliance (DNA appliance®, Vivos Therapeutics, Inc. USA) was prescribed for each subject who declined surgical intervention. All appliances were made following FDA approved protocols and materials, by an FDA-registered orthodontic laboratory. BOAT aims to mimic natural growth and development of the maxillofacial complex, and the system is designed to correct craniofacial and upper airway (nasal cavity to retroglossal region) issues in both children and adults [12-14]. The device used in this study had: 6 anterior 3D axial springs; midline anterior and/or posterior jackscrews; occlusal pads; retentive clasps, and a labial bow (Figure 1). All subjects were instructed to wear the device during the late afternoon, early evening and at nighttime (for approx. 12-16hrs. in total), but not during the daytime and not while eating, partly in line with the circadian rhythm of tooth eruption [15], although this only occurs in children. Adjustments to the devices were performed with 0.25 mm activation as required to optimize their efficacy. Only gentle pressures were transmitted to the teeth, and the functionality of the device was checked with the subject activating a mild force on biting. The appliance was adjusted by the clinician to fit the occlusion of the lower teeth approx. every 4 weeks when all subjects reported for review. At each monthly follow-up, examination for the progress of midfacial development was recorded.

Figure 1. The biomimetic device design used in this study with 6 (patented) anterior 3D axial springs, midline 3-way jackscrew, occlusal pads, retentive clasps, and a labial bow.
For 3D reconstruction, validated software was implemented (Morpheus3D, Morpheus Co. Ltd., Seoul, South Korea). After midfacial segmentation was completed from the CT scans, the minimum transpalatal bone width at the cervical margin of the mesio-palatal cusp of the first molar (Figure 2) was measured prior to and after the end of treatment using BOAT. The angle of the upper central incisor to the cranial base was also measured on the mid-sagittal CBCT slices. The minimum inferior nasal concha distance from nasal septum was measured on the right and left sides. The area of the posterior nasal apertures in the coronal plane was also calculated (Figure 3), and the soft palate length from the posterior nasal spine to the tip of the uvula was measured (Figure 4). Similarly, the minimum antero-posterior retropalatal airway distance was measured (Figure 4); the minimum medio-lateral retropalatal airway distance was measured in the axial plane at the same level (Figure 5), and the minimum retropalatal airway area was then calculated (Figure 5). The minimum antero-posterior retroglossal airway distance was also measured on the mid-sagittal CBCT slices (Figure 4); the medio-lateral retroglossal airway width was measured in the axial plane at the same level (Figure 6), and then the retroglossal airway area in the axial plane was calculated (Figure 6) prior to and after the end of treatment using BOAT with no device in the patient’s mouth.

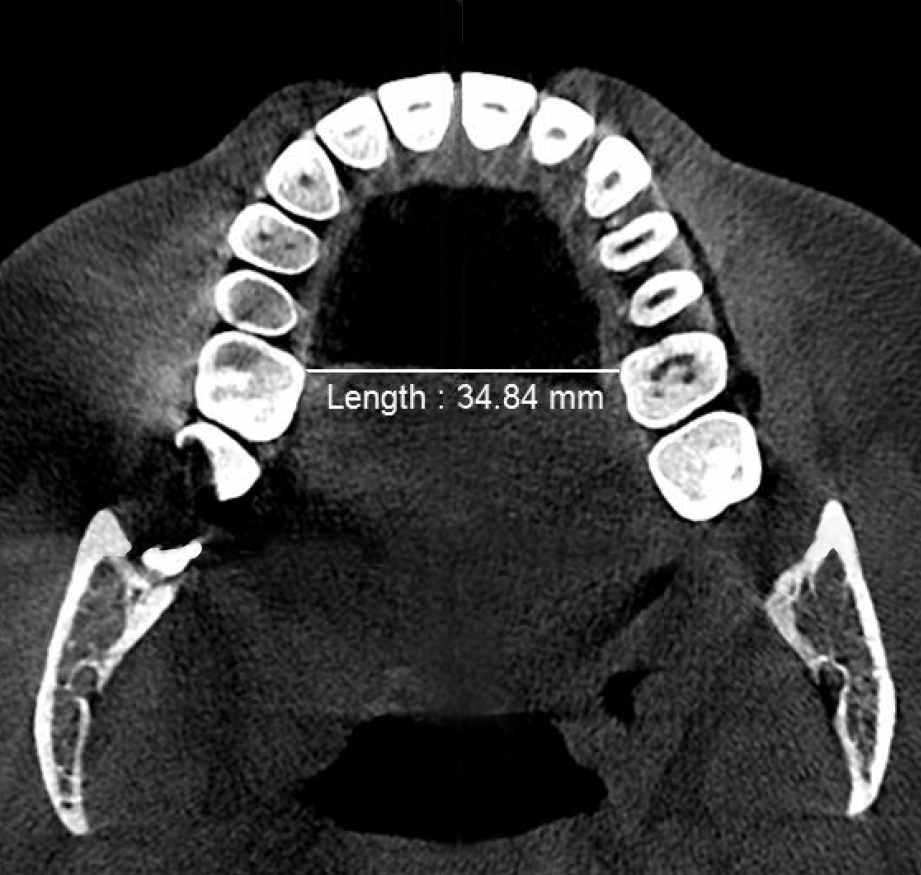
Figure 2a. Pre-treatment minimum transpalatal bone width at the cervical margin of the mesio-palatal cusp of the first molar ≈35mm measured on the CBCT scan prior to biomimetic oral appliance therapy.
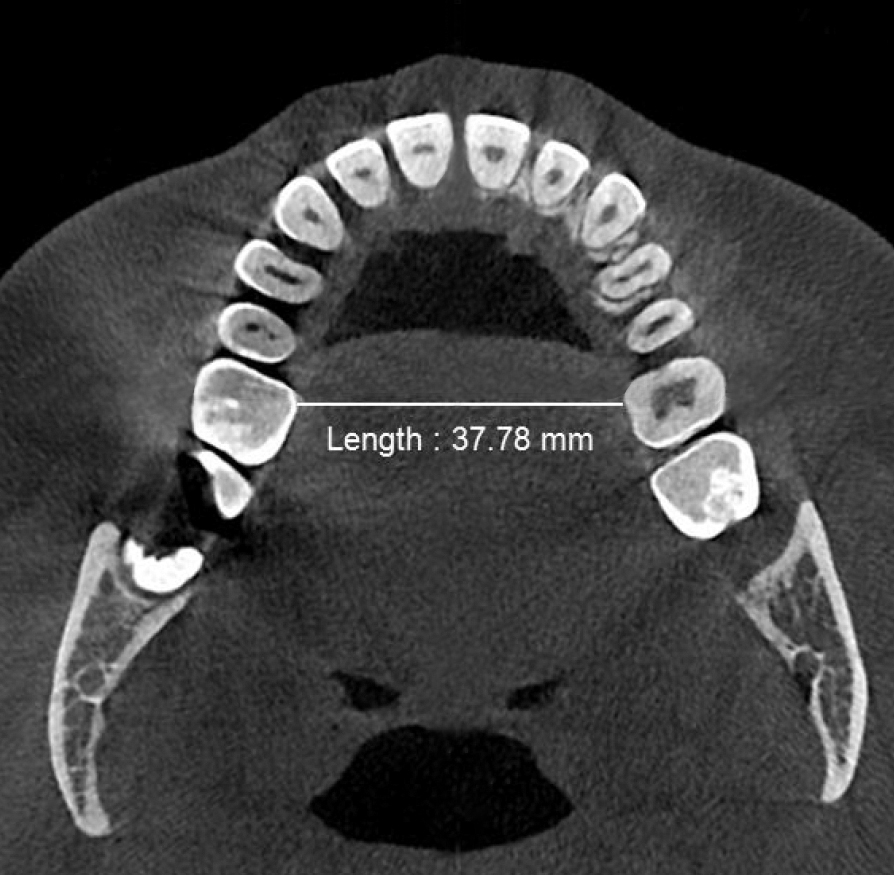
Figure 2b. Post-treatment minimum transpalatal bone width at the cervical margin of the mesio-palatal cusp of the first molar ≈38mm measured on the CBCT scan after biomimetic oral appliance therapy.
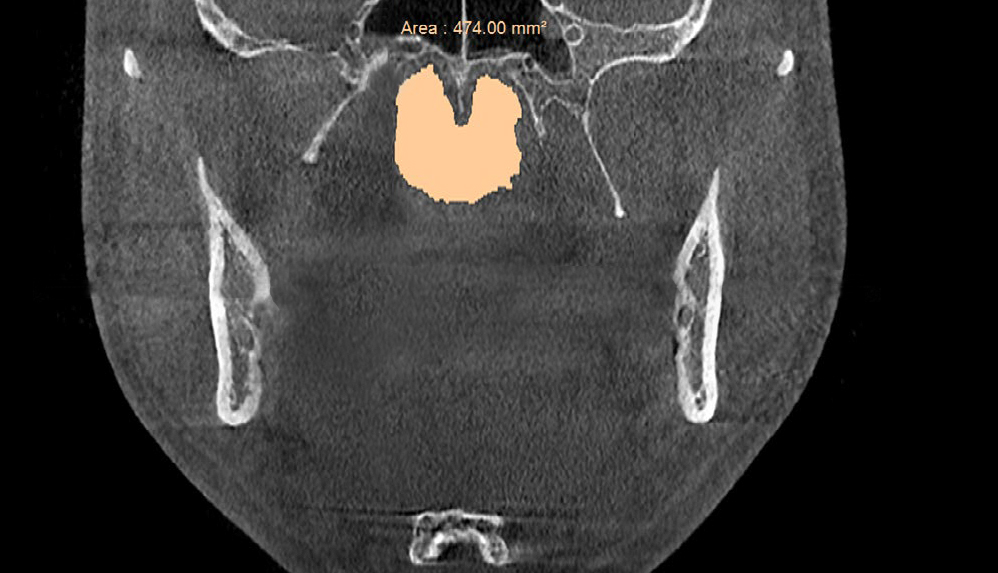
Figure 3a. Pre-treatment area of the posterior nasal apertures in the coronal plane ≈474 mm2 measured on the CBCT scan prior to biomimetic oral appliance therapy.
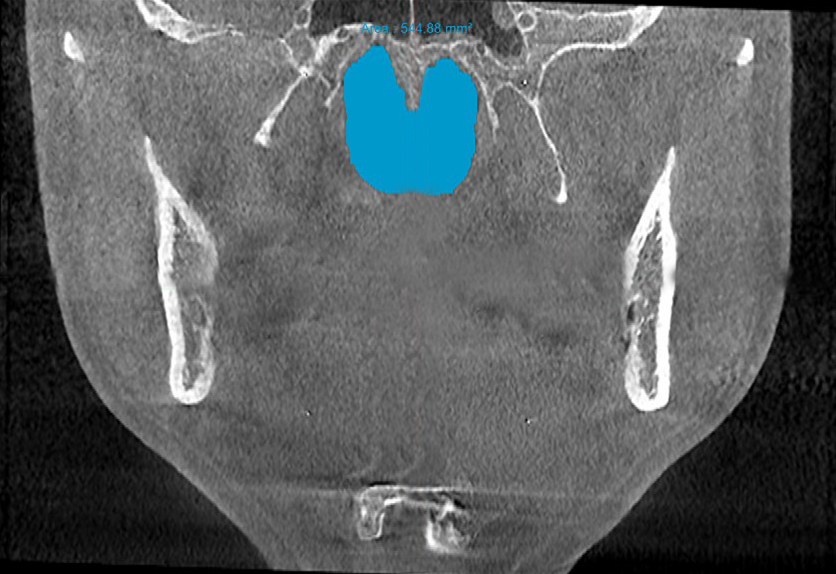
Figure 3b. Post-treatment area of the posterior nasal apertures in the coronal plane ≈545 mm2 measured on the CBCT scan after biomimetic oral appliance therapy.
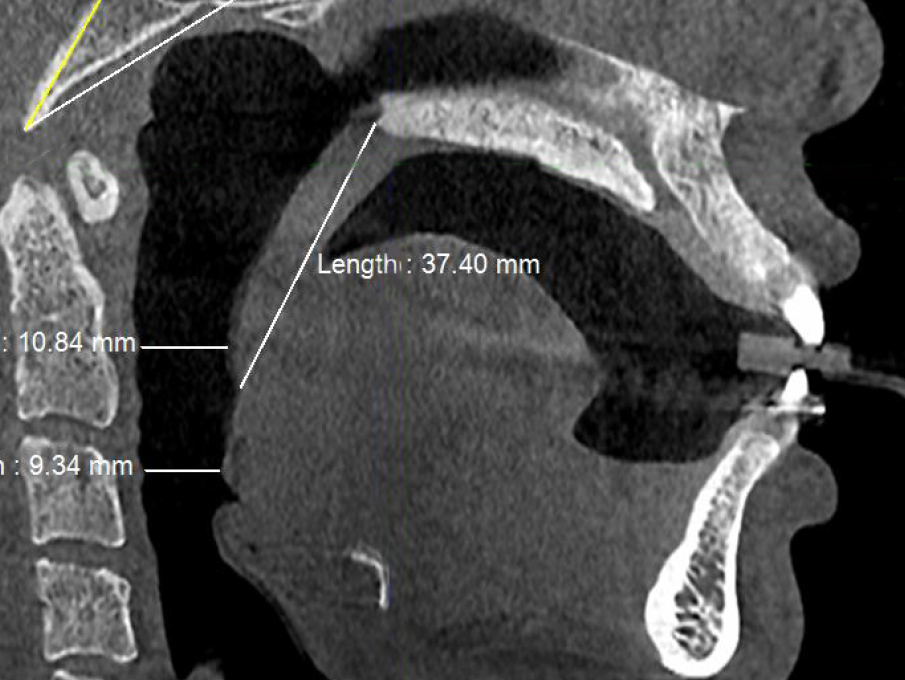
Figure 4a. Pre-treatment CBCT scan in sagittal plane showing minimum retropalatal airway distance (≈11 mm), soft palate length (≈37 mm) and minimum retroglossal airway distance (≈9 mm) with no device in the patient’s mouth.
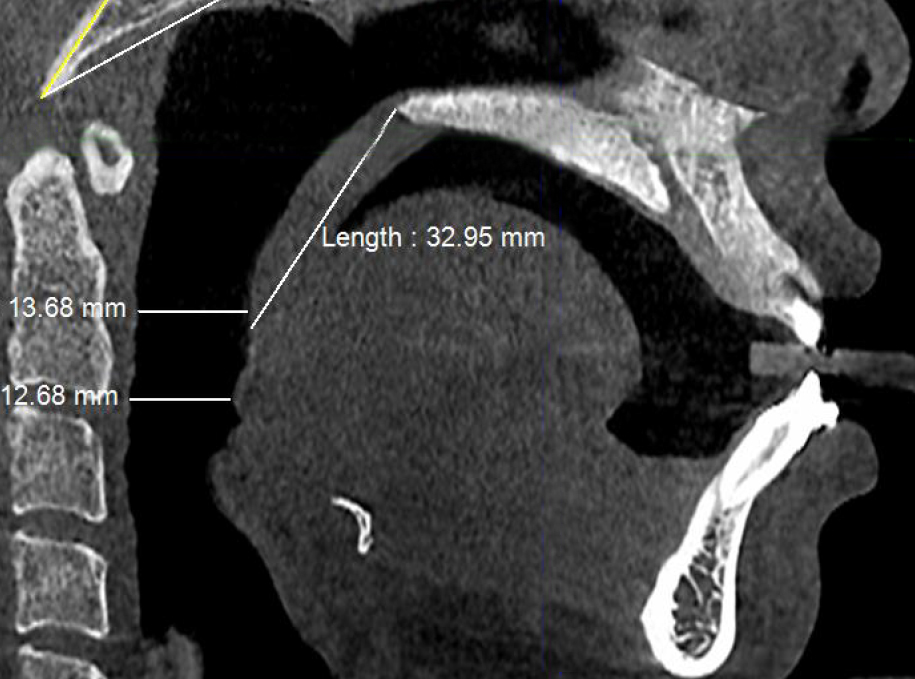
Figure 4b. Post-treatment CBCT scan in sagittal plane showing minimum retropalatal airway distance (≈13.5 mm), soft palate length (≈33 mm) and minimum retroglossal airway width (≈12.5 mm) with no device in the patient’s mouth.
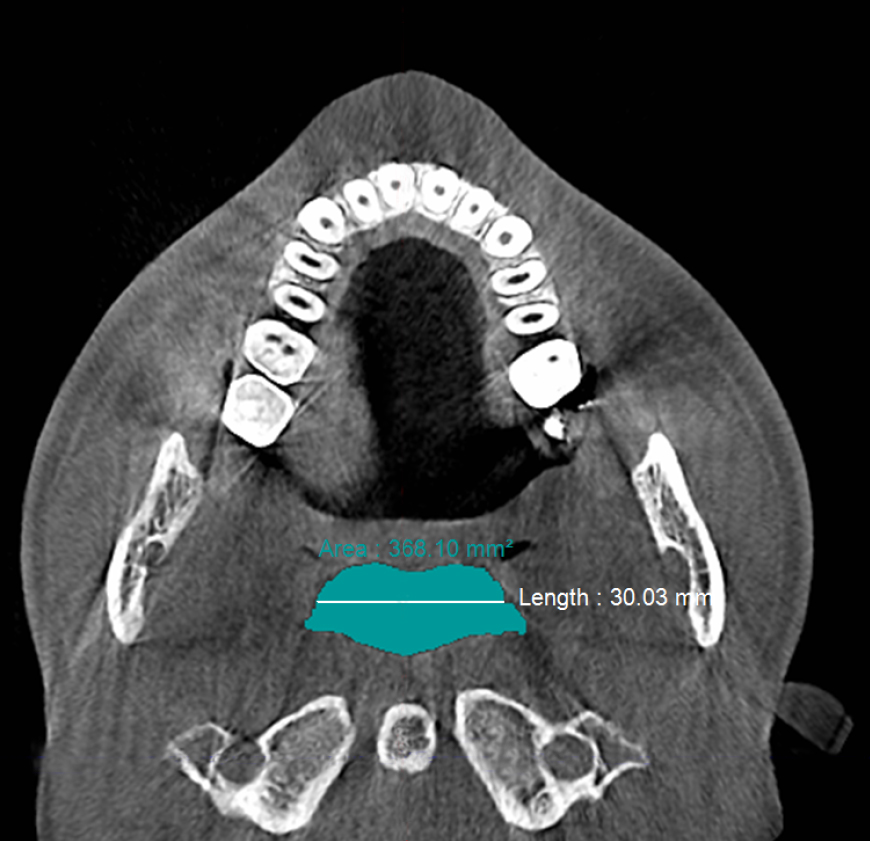
Figure 5a. Pre-treatment CBCT scan in axial plane showingretropalatal width of ≈30 mm and retropalatal area of ≈368 mm2 with no device in the patient’s mouth prior to biomimetic oral appliance therapy.
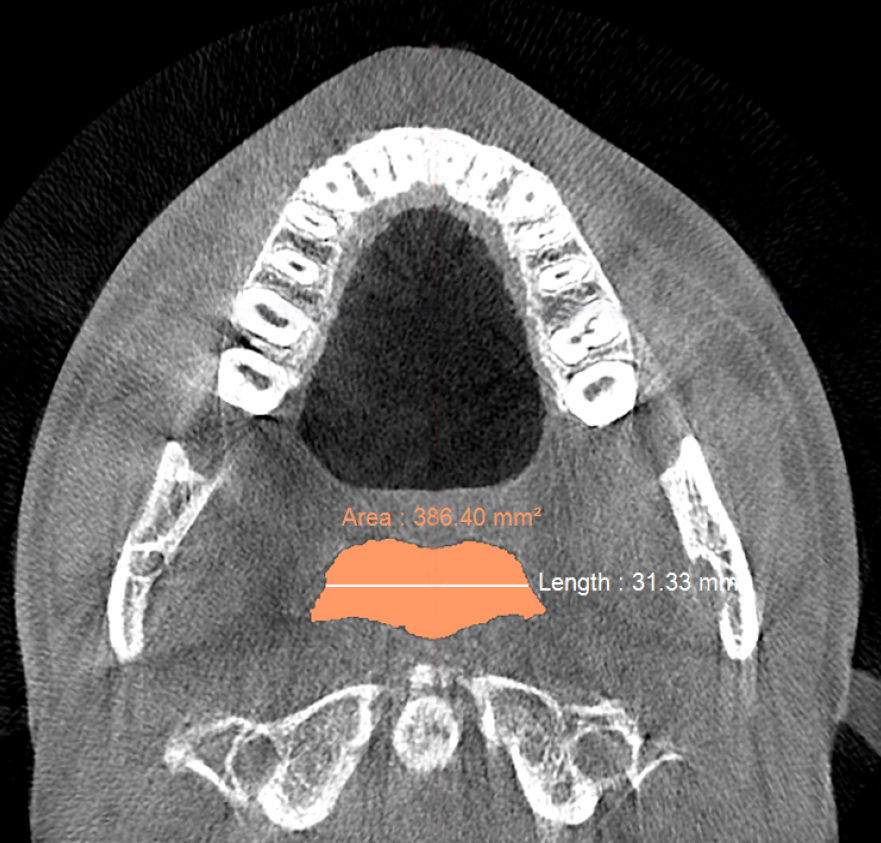
Figure 5b. Post-treatment CBCT scan in axial plane airway showing retropalatal width of ≈31 mm and retropalatal area of ≈386 mm2 with no device in the patient’s mouth after biomimetic oral appliance therapy.
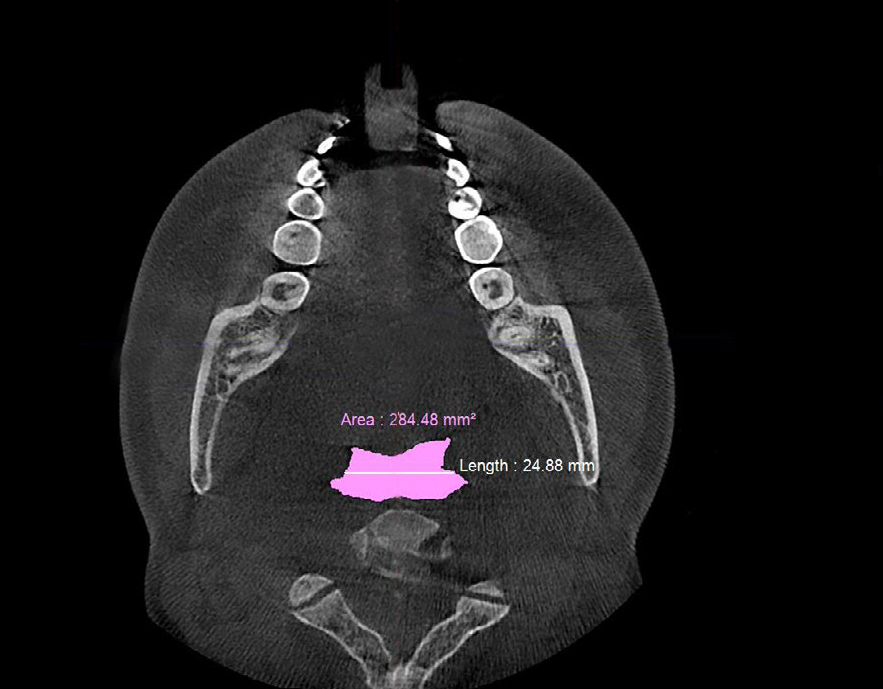
Figure 6a. Pre-treatment CBCT scan in axial plane showing retroglossal width of ≈25 mm and retroglossal area of ≈284.5 mm2 with no device in the patient’s mouth prior to biomimetic oral appliance therapy.
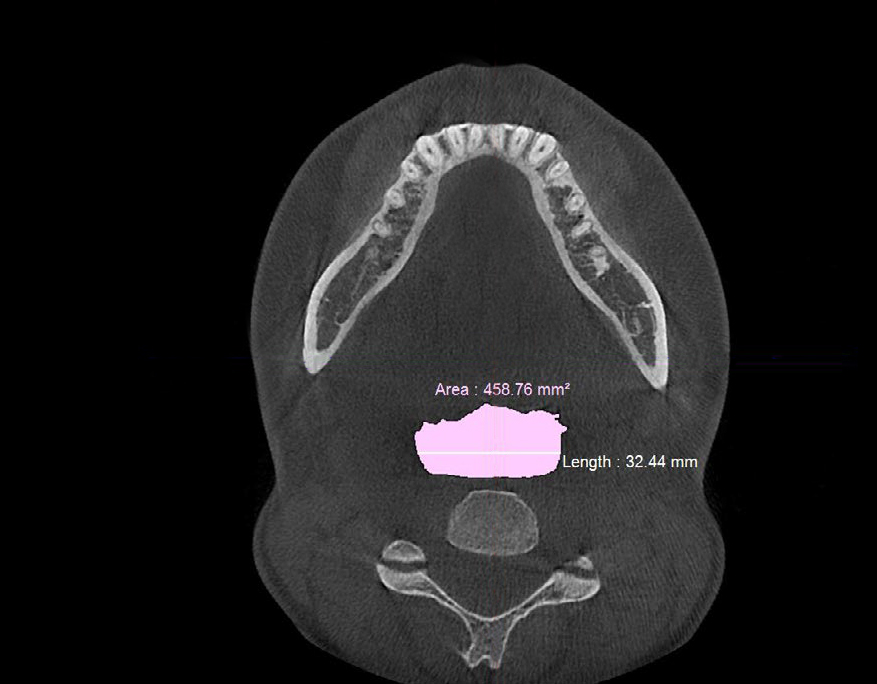
Figure 6b. Post-treatment CBCT scan in axial plane showing retroglossal width of ≈32 mm and retroglossal area of ≈458.5 mm2 with no device in the patient’s mouth after biomimetic oral appliance therapy.
To acquire the nasal cavity volume, the CBCT scan was trimmed anteriorly from nasion to the anterior nasal spine (Figure 7a). The superior aspect was cropped from nasion to the most superior point of the sphenoidal air sinus. The posterior aspect was trimmed from the most superior point of the sinus to its most anterior point, and then to its most posterior point. The inferior aspect was then cropped to the posterior nasal spine. Next, the nasopharynx was trimmed out of the remaining volume, and laterally, the maxillary sinuses were trimmed out at their junction to the nasal cavity. Finally, the nasal airway volume was calculated in all cases (Figure 7b). The measurement protocol was repeated three times to determine the percentage measurement error. The findings were then systematically subjected to univariate and multivariate statistical analyses, using appropriate software (SPSS, Cite, USA). Each variable was tested for normality within each group by an Anderson-Darling test. Also, the assumption of equivalence of variance was verified for each variable by Levene's test, and the corresponding means were subsequently compared for equivalence by a paired t-test, and then subjected to multivariate analysis so that the results could be corroborated.
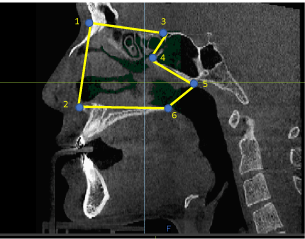
Figure 7a. To acquire the nasal cavity volume, the CBCT scan was trimmed anteriorly from nasion (1) to the anterior nasal spine (2). The superior aspect was trimmed from nasion to the most superior point of the sphenoidal air sinus (3). The posterior aspect was trimmed from the most superior point of the sinus to its most anterior point (4) and then to its most posterior point (5). The inferior aspect was then trimmed to the posterior nasal spine (6).
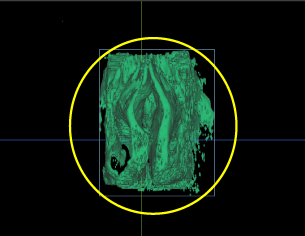
Figure 7b. The nasopharynx was trimmed out of the remaining CBCT volume. Laterally, the maxillary sinuses were trimmed out at their junction to the nasal cavity and, finally, the nasal cavity volume was calculated in all cases.
The medical history, including any medications, of the adults in this study did not preclude orthodontic treatment. All of the subjects were non-obese (mean BMI 21.0 kgm-2 ± 2.1), and none failed to complete the protocol or reported any unwanted side effects such as tooth pain, TMJ issues, etc. The mean age of the final sample was 29.7 yrs ± 8.6 (7 females; 7 males) and the mean treatment time was 16.6 mths ± 4.9. There were no reports of significant weight change or seasonal inflammation in any of the patients during the treatment time. The 3D measurement error was found to be 0.9%, and statistical analysis was warranted. The findings from the Anderson-Darling test showed that the measures were normally distributed. Similarly, Levene's test indicated equivalence of variance for the parameters measured. Therefore, the assumptions of normality and equivalence of variance were accepted, enabling further univariate and multivariate analyses on the pre- and post-treatment findings.
Post-treatment, the transpalatal bone width increased by 9% from 35.6 mm ± 3.1 to 38.7 mm ± 2.1 (p = 0.001), and the mean nasal cavity volume increased by 14% from 22.4 cm3 ± 4.4 to 25.6 cm3 ± 6.0 (p = 0.002) after BOAT. On the other hand, the angle of the upper central incisor to the cranial base remained unchanged (86.00 vs. 88.80 post-treatment) as well as the area of the posterior nasal apertures (530.6 mm2 vs. 510.1 mm2), the soft palate length (35.3 mm vs. 33.4 mm), and the minimum inferior nasal concha distance from nasal septum on the right but not on the left (p = 0.04). Nevertheless, the minimum retropalatal distance in the sagittal plane increased by 25% from 9.9 mm ± 2.7 to 12.4 mm ± 2.1 post-treatment (p = 0.002), while the minimum retropalatal width in the medio-lateral plane remained unchanged (26.4 mm to 27.9 mm post-treatment). However, the minimum retropalatal area in the axial plane at the same level increased by 24% from 256.9 mm2 ± 95.6 to 318.5 mm2 ± 115 (p = 0.017). Finally, the minimum sagittal retroglossal distance increased by 20% from 11.3 mm ± 3.5 to 13.6 mm post-treatment ± 2.4 (p = 0.007); the minimum medio-lateral retroglossal width increased by 11% from 23.8 mm ± 4.5 to 26.4 mm ± 5.5 post-treatment (p = 0.026), while the minimum retroglossal area in the axial plane at the same level increased by 25% from 281.3 mm2 ± 89.8 to 351.4 mm2 ± 118 (p = 0.017) with no device in the patient’s mouth. The multivariate tests also showed a significant difference for the treatment effect on the upper airway parameters (p = 0.012). These results are summarized in Table 1 and Figures 2-7.
Table 1. Craniofacial parameters measured in this study. Pre: Pre-treatment; Post: Post-treatment; Std. Dev.: Standard Deviation.
The aim of this study was to localize and quantify changes in 3D craniofacial and upper airway architecture using BOAT as a novel approach to investigate the degree of upper airway plasticity and remodeling that resides in the system. However, in this retrospective study, various parameters could limit the generalizability of the present findings through selection bias, since the subjects included were of a limited age range and were recruited from a specific clinical population of patients with no control group, which is similar to other studies of this nature. In addition, only those subjects that completed the protocol were included, which is a potential source of attrition bias [16]. Nevertheless, this study tested one aspect of the Spatial matrix hypothesis [17] in that palatal widening represents a change in spatial relations, which is thought to initiate a cascade of events that subsequently yield structural modifications of the phenotype until the system regresses to a new level of craniofacial homeostasis. This epigenetic synthesis might be achieved through the genetic potential of an individual in that localized stem cell populations are signaled to produce a more optimal outcome of the craniofacial architecture in the new conditions, observed as quantifiable changes in upper airway morphology.
To effect upper airway changes in cases of OSA, there has long been an interest in the effects of rapid maxillary expansion (RME). Cistulli et al. [18] were the first to study the effect of RME in a sample of 10 adults (mean age 27 yrs) with mild to moderate OSA, about half of which required additional surgical assistance. Their preliminary data suggested that partially surgically assisted RME (SARME) may be useful in the treatment of OSA in adult patients. Therefore, it not surprising that in our present study we found that non-surgical BOAT improved the transpalatal bone width by 9% from 35.6 mm ± 3.1 to 38.7 mm ± 2.1 (p < 0.001), while the angle of the upper central incisor to the cranial base remained unchanged, suggesting that this width increase was not attributed to simply tipping the teeth. Indeed, there was no evidence of either dehiscence or fenestration of the buccal cortical plates. Given that the horizontal plate of the palatine bone forms not only the roof of the mouth but also the floor of the nose, we also found that BOAT produced an increase in nasal cavity volume by 14% from 22.4 cm3 ± 4.4 to 25.6 cm3 ± 6.0 (p < 0.01). This finding is corroborated by an earlier study [19] that reported a 5% increase in nasal airway volume using BOAT in Caucasians. On the other hand, in our present study, the area of the posterior nasal apertures remained unchanged, indicating that the localization of nasal changes may be determined by stem cell containing sutural sites [20] further anteriorly within the hard palate. This contention is further supported by our finding that the distance between the nasal septum and inferior conchae remained relatively unchanged, as did the soft palate length (Table 1). Haralambidis et al. [21] investigated changes of the nasal cavity after RME, using CT scans of Turkish children. After expansion, they reported a 11% increase in nasal volume, which helped promote nasal respiration. Similarly, using acoustic rhinometry, De Felippe et al. [22] found that RME enlarged the nasal valve area, increased the nasal cavity volume and decreased upper airway resistance. Furthermore, in a systematic review, Gordon et al. [23] evaluated the effects of RME on nasal airway cross-sectional area and nasal volume with acoustic rhinometry. They concluded that while there were some small increases in nasal cavity volume compared to controls, these increases were less stable in adult patients who were not actively growing at the time of intervention. Because of these types of concerns, Tausche et al. [24] deployed SARME in adults. In a sample of 17 patients (mean age 29 yrs), they showed a 5% increase in nasal volume using CT scans 6 months after SARME. In a similar study on a sample of 13 patients (mean age 31 yrs), Seeberger et al. [25] found a 23% enhancement of nasal volume in patients associated with SARME using acoustic rhinometry. Thus, deformational plasticity or remodeling appears to be a characteristic feature of the nasal airway, at least to some extent.
It is also thought that both craniofacial size and obesity can alter upper airway size [26]. In that respect, in addition to nasal airway morphology, Avci et al. [27] consider it important that features of the retropalatal airway area are assessed during the treatment planning of cases with OSA. Specifically, their study indicated that the retropalatal area and width at the level of the hard palate were associated with OSA. In fact, in patients with syndromic craniosynostoses, Resnick et al. [28] found that a co-diagnosis of OSA was associated with a smaller retropalatal cross-sectional area with a high degree of both sensitivity and specificity. While it can be inferred that this specific subpopulation would not be amenable to sutural remodeling using BOAT, our study excluded craniofacial anomalies as well as obesity. Accordingly, we found that the retropalatal airway distance in the sagittal plane increased by 25% from 9.9 mm ± 2.7 to 12.4 mm ± 2.1 post-treatment (p < 0.001), while the minimum retropalatal area in the axial plane at the same level increased by 24% from 256.9 mm2 ± 95.6 to 318.5 mm2 ± 115 (p < 0.05). On the other hand, both the retropalatal width in the medio-lateral plane and the soft palate length remained unchanged, indicating an anisotropic pattern of deformation, which presumably reflects varying velopharyngeal behavioral patterns [29].
Another interesting finding in our present study is that BOAT, which is centered around midfacial redevelopment, appeared to have an effect on the retroglossal airway. Specifically, in the sagittal plane, the retroglossal distance increased by 20% from 11.3 mm ± 3.5 to 13.6 mm post-treatment ± 2.4 (p < 0.01) and the minimum medio-lateral retroglossal width increased by 11% from 23.8 mm ± 4.5 to 26.4 mm ± 5.5 post-treatment (p < 0.05), while the minimum retroglossal area increased by 25% from 281.3 mm2 ± 89.8 to 351.4 mm2 ± 118 (p < 0.05) with no device in the patient’s mouth. These findings highlight the essential difference between BOAT and conventional mandibular advancement devices (MADs). While MADs mechanically enlarge the airway while being worn in situ [30], BOAT aims to mimic natural craniofacial growth and development to induce putative upper airway remodeling that persists even after the device has been removed. According to the cranio-caudal gradient of development, the functional space of the mandible will largely be dictated by the maxilla, and thus maxillary deficiency likely precipitates mandibular retrognathia [31] with concomitant effects on the retroglossal airway. In fact, Yang et al. [32] showed in a sample of 12 Korean adults that surgical mandibular setback procedures decrease the retroglossal area and significantly increased the AHI. In contrast, our multivariate tests also showed a significant (p = 0.012) non-surgical improvement through BOAT’s treatment effect on the upper airway, including the retroglossal area. Therefore, our findings lead us to conclude that imbalances of the craniofacial architecture affect upper airway morphology and these deficiencies might be targeted using a biomimetic approach in the treatment of OSA. Nevertheless, the results of this present study are based on non-obese adult orthodontic patients with midfacial hypoplasia and might not be generalizable to those diagnosed with OSA. In view of this concern, a larger clinical trial is required to determine the role of BOAT in patients with midfacial hypoplasia and sleep disordered breathing. Thus, craniofacial sleep medicine remains in its infancy and the relationship of putative upper airway remodeling and its effect on functional nocturnal respiratory behaviors requires further scientific investigation.
These findings suggest that craniofacial architecture and upper airway morphology can be non-surgically enhanced in non-growing adults.
We would like to thank Kingman Strohl, MD, Division of Pulmonary Critical Care and Sleep Medicine, University Hospital, Cleveland, OH for his advice on this project. All devices used in this study were fabricated by STAA Orthodontic Laboratory, Seoul, South Korea.
- 1. Enache AM, Nimigean VR, Mihălţan F, Didilescu AC, Munteanu I, et al. (2010) Assessment of sagittal and vertical skeletal patterns in Romanian patients with obstructive sleep apnea. Rom J Morphol Embryol 51: 505-508. [Crossref]
- 2. Dobrowolska-Zarzycka M, Dunin-Wilczyńska I, Szymańska J (2016) Craniofacial structure in patients with obstructive sleep apnoea. Folia Morphol (Warsz) 75: 311-315. [Crossref]
- 3. Lam B, Ip MSM, Tench E, Ryan CF (2005) Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax 60: 504-510. [Crossref]
- 4. Banabilh SM Suzina AH, Dinsuhaimi S, Singh GD (2009) Craniofacial obesity in patients with obstructive sleep apnea. Sleep Breath 13: 19-24. [Crossref]
- 5. Wysocki J, Charuta A, Kowalcze K, Ptaszyńska-Sarosiek I (2016) Anthropometric and physiologic assessment in sleep apnoea patients regarding body fat distribution. Folia Morphol (Warsz) 75: 393-399. [Crossref]
- 6. Yilmaz A, Akcaalan M (2017) What can anthropometric measurements tell us about obstructive sleep apnoea? Folia Morphol (Warsz) 76: 301-306. [Crossref]
- 7. Rivlin J, Hoffstein V, Kalbfleisch J, McNicholas W, Zamel N, et al. (1984) Upper airway morphology in patients with idiopathic obstructive sleep apnea. Am Rev Respir Dis 129: 355-360. [Crossref]
- 8. Singh GD, Olmos S (2007) Use of a sibilant phoneme registration protocol to prevent upper airway collapse in patients with TMD. Sleep Breath 11: 209-216.
- 9. Krasny M, Wysocki J, Prus M, Niemczyk K (2017) Location of the narrowest area of the pharynx regarding body mass index and obstructive sleep apnoea severity. Folia Morphol (Warsz) 76: 491-500.
- 10. Xu L, Keenan BT, Wiemken AS, Chi L, Staley B, et al. (2019) Differences in three-dimensional upper airway anatomy between Asian and European patients with obstructive sleep apnea. Sleep 43: zsz273. [Crossref]
- 11. Cooper BC, Cooper DL, Lucente FE (1991) Electromyography of masticatory muscles in craniomandibular disorders. Laryngoscope 101:150-157. [Crossref]
- 12. Heit T, Sebastian J, Singh GD (2016) A novel combined protocol for the resolution of severe obstructive sleep apnea. J Sleep Disord Ther 5: 251-254.
- 13. Singh GD, Kraver M, Chernyshev O (2019) Restoration of sleep using a novel biomimetic protocol for adult OSA: Clinical case report. Cranio 37: 136-139. [Crossref]
- 14. Hwang H, Hwang C, West J, Singh GD (2019) Changes in pediatric paranasal sinuses following biomimetic oral appliance therapy: 3 case reports. Cranio 2: 1-6. [Crossref]
- 15. Proffit WR, Frazier-Bowers SA (2009) Mechanism and control of tooth eruption: overview and clinical implications. Orthod Craniofac Res 12: 59-66. [Crossref]
- 16. Dort LC (2017) Bias in dental sleep medicine research: does it matter to clinicians? J Dent Sleep Med 4: 55.
- 17. Singh GD (2004) On Growth and Treatment: The spatial matrix hypothesis. Craniofacial Growth Series. Ann Arbor, 41: 197-239.
- 18. Cistulli PA, Palmisano RG, Poole MD (1998) Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep 21: 831-835. [Crossref]
- 19. Singh GD, Heit T, Preble D, Chandrashekhar R (2016) Changes in 3D nasal cavity volume after biomimetic oral appliance therapy in adults. Cranio 34: 6-12. [Crossref]
- 20. Zhao H, Feng J, Ho TV, Grimes W, Urata M, et al. (2015) The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol 17: 386-396.
- 21. Haralambidis A, Ari-Demirkaya A, Acar A, Küçükkeleş N, Ateş M, et al. (2009) Morphologic changes of the nasal cavity induced by rapid maxillary expansion: a study on 3-dimensional computed tomography models. Am J Orthod Dentofacial Orthop 136: 815-821. [Crossref]
- 22. De Felippe NL, Bhushan N, Da Silveira AC, Viana G, et al. (2009) Long-term effects of orthodontic therapy on the maxillary dental arch and nasal cavity. Am J Orthod Dentofacial Orthop 136: 490.e1-8. [Crossref]
- 23. Gordon JM, Rosenblatt M, Witmans M, Carey JP, Heo G, et al. (2009) Rapid palatal expansion effects on nasal airway dimensions as measured by acoustic rhinometry. A systematic review. Angle Orthod 79: 1000-1007. [Crossref]
- 24. Tausche E, Deeb W, Hansen L, Hietschold V, Harzer W, et al. (2009) CT analysis of nasal volume changes after surgically-assisted rapid maxillary expansion. J Orofac Orthop 70: 306-317. [Crossref]
- 25. Seeberger R, Kater W, Davids R, Thiele OC (2010) Long term effects of surgically assisted rapid maxillary expansion without performing osteotomy of the pterygoid plates. J Craniomaxillofac Surg 38: 175-178.
- 26. Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T (2002) Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med 165: 260-265. [Crossref]
- 27. Avci S, Lakadamyali H, Lakadamyali H, Aydin E, Tekindal MA (2019) Relationships among retropalatal airway, pharyngeal length, and craniofacial structures determined by magnetic resonance imaging in patients with obstructive sleep apnea. Sleep Breath 23:103-115. [Crossref]
- 28. Resnick CM, Middleton JK, Calabrese CE, Ganjawalla K, Padwa BL (2019) Retropalatal cross-sectional area is predictive of obstructive sleep apnea in patients with syndromic craniosynostosis. Cleft Palate Craniofac J 24: 1055665619882571. [Crossref]
- 29. Witzel MA, Posnick JC (1989) Patterns and location of velopharyngeal valving problems: atypical findings on video nasopharyngoscopy. Cleft Palate J 26: 63-67. [Crossref]
- 30. Strollo PJ Jr, Rogers RM (1996) Obstructive sleep apnea. N Engl J Med 334: 99-104. [Crossref]
- 31. McNamara JA Jr (1981) Components of Class II malocclusion in children 8-10 years of age. Angle Orthod 51: 177-202. [Crossref]
- 32. Yang HJ, Jung YE, Kwon IJ, Lee JY, Hwang SJ (2020) Airway changes and prevalence of obstructive sleep apnoea after bimaxillary orthognathic surgery with large mandibular setback. Int J Oral Maxillofac Surg 49: 342-349. [Crossref]