Abstract
Objective: To analyze the lipid composition of coronary endarterectomy plaques and explore the mechanisms affecting the mid- and long-term efficacy of coronary endarterectomy (CE).
Methods: A total of 50 patients with diffuse coronary artery disease (DCAD) with a history of hyperlipidemia before cardiac surgery that was performed in the First Affiliated Hospital of Guangxi Medical University between January 2018 and December 2019 were selected. Patients underwent coronary artery bypass grafting combined with anterior descending artery endarterectomy (CE). Intraoperative coronary endarterectomy and plasma samples were taken from all patients. After the re-examination of magnetic resonance imaging (MRI) coronary plaque imaging (Atherosclerosis T1-weighted Characterization, CATCH) and Power Domain Non-Orthogonal Multiple Access (NOMA) integrated framework technology analysis of the long-term coronary restenosis rate, a high-risk group (>25%, study group) and a matched low-risk group (control group) were set for lipidology and molecular biology analysis to detect plaque tissue and cytochrome P450 3A4 enzyme (CYP3A4) content.
Results: Eight patients were selected from each group. The lipidomics analysis of coronary endarterectomy plaque in the study group showed that the content of 4α-hydroxycholesterol (4α-Hydroxy cholesterol, 4αOH-CHO) was significantly higher than that in the control group (0.050umol/g vs. 0.016umol/g, P<0.05). At the same time, the re-examination of coronary T1-weighted characteristic MRI results 12 months after operation showed that the patency rate was better in the control group compared to the study group [coronary stenosis rate (9.0 ± 1.9) % vs. (22.3 ± 2.3)%, P<0.05)]. The plasma CYP3A4 levels in the study group were higher than those in the control group [immediately (0.88 ± 0.05) ng/ml vs (0.45 ± 0.03) ng/ml, 12 months after surgery (2.08 ± 0.40) ng/ml vs (1.58) ng/ml vs. ± 0.16) ng/ml, P<0.05)].
Conclusion: High expression of 4αOH-CHO may accelerate atherosclerosis (AS) and cause mid- and long-term restenosis after CE. Also, 4αOH-CHO was confirmed as a biological marker of CYP3A4, thus laying a foundation for assessing the AS progression mechanism after CE.
Keywords
4α-hydroxycholesterol, cytochrome P450 3A4 enzyme, coronary atherosclerosis, MRI coronary plaque imaging, power domain non-orthogonal multiple access
Introduction
Diffuse coronary artery disease (DCAD) is a critical type of coronary heart disease (CAD) with high mortality and difficult revascularization. Coronary endarterectomy (CE) is an effective surgical method for the treatment of DCAD; however, its postoperative vascular occlusion rate is high, which can have a serious effect on surgical efficacy. During CE operation, the operator manually peels off the thickened coronary intima, after which the coronary media and its fibroblasts, extracellular matrix, elastic fibers, etc. are exposed to the arterial blood flow environment for a long time, which tends to increase the number of new risks of atherosclerosis (AS) plaque formation. Therefore, slowing down the process of AS has become the focus of interest for preventing restenosis after CE.
Lipid metabolism is an essential factor in AS, and the regulation of lipid synthesis and metabolism after CE is one of the main research interests. In addition to statin therapy, the regulation of AS processes should also be analyzed at the molecular biological level. This study innovatively used the difference in restenosis rate after CE, reversely studied the difference of oxidized cholesterol in patients, carried out morphological, lipid, and molecular biological analysis, and conducted in-depth research on the occurrence and development of AS after CE, which is helpful to clinically prevent restenosis after CE.
Materials and Methods
A total of 50 inpatients with a history of hyperlipidemia before cardiac surgery in the First Affiliated Hospital of Guangxi Medical University were selected between January 2018 and December 2019. Inclusion criteria were the following: preoperative coronary angiography showed multiple-vessel lesions with severe calcification, anterior descending artery occlusion/severe stenosis, and patients who were planned to undergo coronary artery bypass grafting plus anterior descending coronary endarterectomy [1]. Exclusion criteria were: severe cardiac insufficiency (NYHA class of cardiac function ≥ Ⅲ or left ventricular ejection fraction < 0.35), severe hepatic and renal insufficiency, severe hyperlipidemia with poor preoperative drug control or combined with acute and chronic infection.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University [No. 2020-KT-263]. All patients signed preoperative informed consent.
Intraoperative endarterectomy of the anterior descending coronary artery was immediately performed, and the blood impurities were washed with sterile saline, after which they were placed in a sterile specimen tube and transferred to a liquid nitrogen tank for storage. Immediately during the operation and after the re-examination, 2 ml of peripheral blood was extracted from the patient, and the centrifuged supernatant was the total plasma protein, which was added to the loading buffer and stored in liquid nitrogen.
All patients underwent CATCH examination 12 months after surgery based on the results of previous studies [2] and combined NOMA technology for innovative AS imaging analysis [3]. CATCH technology can capture and accurately locate small AS after CE in the anterior descending artery, while NOMA technology Coronary stenosis rate is calculated by AS maximum area/coronary lumen area. Patients with coronary artery stenosis rate > 25% were defined as high risk and were included in the research group (8 cases in total). The following informations were collected: the preoperative data of patients, gender, age, diabetes, hypertension, preoperative myocardial infarction history, smoking history, ventricular ejection fraction, peripheral vascular disease, and the number of bypass grafts. Meanwhile, matched low-risk patients were enrolled in the control group. The exfoliated plaque samples of the two groups were analyzed by lipidomics to compare the differences in oxidized cholesterol between the two groups. The plasma of the patients was also analyzed by ELISA to compare the differences in CYP3A4 content.
The lipidomic analysis in the present study was based on a modified tissue extraction method used to extract cholesterol from plaque samples with the addition of an appropriate internal standard [4]. All analyses were performed using an Exion UPLC-QTRAP 6500 PLUS (Sciex) LC/MS in electrospray ionization (ESI) mode with the following conditions: curtain gas = 20 psi, ion spray voltage = 5500 V, temperature = 400 °C, ion source Gas 1 = 35psi, ion source gas 2 = 35psi. Free cholesterol and sterols and corresponding esters were analyzed by liquid chromatography-tandem mass spectrometry, atmospheric pressure chemical ionization mode (APCI), and quantified using cholesterol-26,26,26,4α,4β,4α-d6 as internal standard. Multivariate statistical analysis was performed, and the original maps and data obtained from the analysis were imported into EZinfo 2.0 software for analysis, including principal component analysis (PcA) and orthogonal least squares discriminant analysis (OPLS-DA).
During CE operation and re-examination, 2ml of venous blood was collected from the two groups of patients, then centrifuged and placed in a sterile centrifuge tube for extraction of CYP3A4 enzyme protein. Enzyme-linked immunosorbent assay (ELISA) was used to analyze the content of CYP3A4 according to the kit’s instructions.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 statistical analysis software. Metering data is represented by mean ± SD. The lipidomic differences between the two groups were compared using a parametric test paired t-test and a nonparametric test signed-rank sum hypothesis test. P<0.05 was considered statistically significant. R software (v3.6.3) was used for volcano plot and hierarchical clustering analysis.
Results
All the enrolled patients were discharged as there were no perioperative deaths. A total of 46 cases successfully underwent coronary artery bypass grafting + anterior descending artery endarterectomy. Eight patients with a high risk of restenosis (>25%, study group) were screened out after the CATCH+NOMA review after the operation; 8 patients in the low-risk group (control group) were selected after matching the preoperative data. The clinical data of the two groups of patients are compared in Table 1. The preoperative data of the two groups were basically comparable. The number of patients who underwent coronary artery bypass grafting was 5 in the low-risk group and 1 in the high-risk group. There were no significant differences in other preoperative factors between the two groups.
Analysis of lipid metabolism components
According to the dimensionality reduction analysis of the plaque tissue of the experimental group, Figure 1(A) shows the principal component analysis gravel plot; Figure 1(B) and Figure 1(C) show the top 10 variables with the highest loading on the principal component. As shown in the gravel map, the highest content of principal component 1 contributed 42.9%, while the contribution of principal component 2 was 25.2%. Among them, the contribution rate of the top three components sharply dropped, and the cumulative contribution rate was 81%. The subsequent curves showed a smooth trend. Among them, 4αOH-CHO occupied a relatively high proportion in the first two main components.
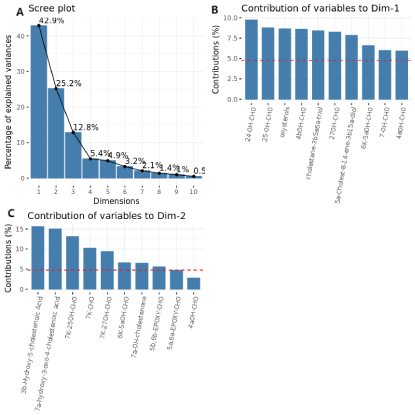
Figure 1. Principal component analysis of the plaques in the experimental group. (A) The principal component analysis rubble diagram shows the contribution ratio of all components. (B, C) The top 10 with the highest loading on the principal components 1 and 2 with the highest contribution ratio variable
A subsequent comparison of the differences in component content between the experimental group and the control group was performed; the clustering heat map is shown in Figure 2. As shown in a heat map, the two groups had different colors, reflecting that the experimental group and the control group had significant differences in the content of components. At the same time, the volcano plot showed the 20 components with the largest differences among the specific differential components, along with their P valuesand fold changes (Figure 3). The figure showed significant differences between the two groups in terms of oxidative sterols, such as 4αOH-CHO and 24-OH-CHO. Also, the content of 4αOH-OHC in the research group was significantly increased. The bar graph (Figure 4) showed that the content of 4αOH-CHO in the experimental group and the control group was (0.050 ± 0.018) umol/g versus (0.016 ± 0.009) umol/g, P=0.003, respectively.
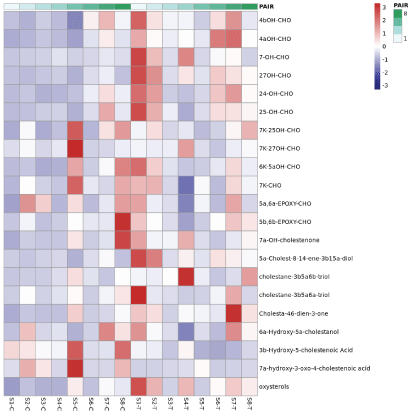
Figure 2. Clustering heat map of the comparative analysis of the component variables of the plaque tissue in the experimental group and the control group. The blue on the left represents the control group, and the red on the right represents the experimental group. Different colors represent the difference in expression level
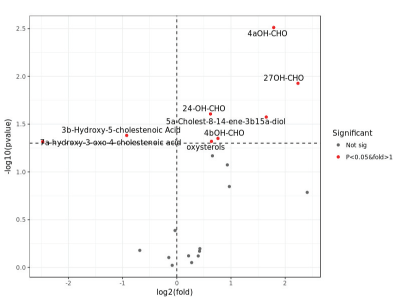
Figure 3. Volcano plot of content differences between the experimental group and the control group for the comparative analysis of component variables in the plaque tissue. The red dots represent the component variables that were significantly differentially expressed between the experimental group and the control group
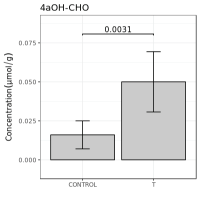
Figure 4. Column chart of the content of 4aOH-CHO in the experimental group versus the control group
Changes in the expression of CYP3A4
ELISA results showed that the plasma CYP3A4 content of the study group was also higher than that of the control group. It was (0.88 ± 0.05) ng/ml in the study group immediately during the operation and (2.08 ± 0.40) ng/ml 12 months after the operation; it was immediately (0.45 ± 0.03) ng/ml in the control group during the operation and (1.58 ± 0.16) ng/ml 12 months after operation; the differences were statistically significant (P<0.05).
CATCH+NOMA Analysis Results
The 12-month postoperative re-examination of coronary T1-weighted magnetic resonance imaging in the study group showed that the patency rate in the control group was (9.0 ± 1.9)% significantly higher than that of the study group (22.3 ± 2.3)% (P<0.05).
Discussion
The main pathological manifestations of mid- and long-term restenosis after CE surgery are coronary medial thickening and the formation of new AS plaques, which substantially limit the surgical effect. At the same time, endothelial cells (EC), as an important histological structure in the arterial wall, exert various protective effects such as anti-inflammatory, which are crucial for maintaining the function of the arterial wall and preventing the occurrence of AS. CE peels off the intima of the lesion, thus causing the loss of the protective effect of the coronary endothelium. The lost protective effect mainly includes the following three points: (1) inability to secrete various anti-inflammatory and relaxation factors; (2) CE regulates the proliferation and secretion of smooth muscle cells in the vessel wall; (3) loss of regulatory effect on lipid metabolism, resulting in increased production of low-density lipoprotein cholesterol (LDL-C) and oxidized cholesterol [5]. Once new AS plaques are formed in bypass vessels or target vessels after CE, LDL-C and oxidized cholesterol reduce plaque stability and increase the incidence of postoperative acute myocardial infarction and long-term restenosis [6].
Many studies have shown that the occurrence of AS is strongly related to the content of cholesterol in the body, thus suggesting that high cholesterol is an independent risk factor for the progression of AS. Meanwhile, long-term survival curve analysis confirmed that hypercholesterolemia is an independent risk factor for long-term restenosis and long-term death after CE surgery [7-9]. The disruption of cholesterol homeostasis can directly promote restenosis in AS. At the same time, it can induce the expression of various inflammatory factors such as tumor necrosis factor (TNF-α), enhance the activity of macrophages, affect the function of adipocytes and inhibit the transfer of blood lipid components into adipocytes. The high cholesterol environment directly inhibits the proliferation of endothelial cells and delays the process of re-endothelialization after CE [10,11]. Dyslipidemia caused by disordered cholesterol metabolism is a recognized cause of AS; however, the effects of various subtypes of oxidized cholesterol on AS have not yet been fully elucidated.
There are many types of oxidized cholesterol, and the mechanisms regulating and maintaining cholesterol homeostasis are complex. Among them, 4α-hydroxycholesterol and 4-hydroxycholesterol (4βOH-CHO) have gained the most attention. 4αOH-CHO, the CYP3A4 metabolite of cholesterol, has been shown to be an important marker for identifying the biological activity of endogenous cholesterol [12,13]. While 4αOH-CHO is an isomer of 4βOH-CHO, relevant studies have rarely reported on it. Human plasma has low concentrations of 4α- and 4β-OHC. In fact, they are lower than in many other oxidized cholesterols and much lower than cholesterol itself [12-14]. Therefore, the results of 4α- and 4β-OHC content in CE specimens are highly specific, and both were highly expressed in the study group, especially 4αOH-CHO, thus suggesting that the high expression of 4αOH-CHO was significantly associated with mid- and long-term AS progression and CE restenosis. At the same time, the plasma CYP3A4 content increased in the research group immediately and 12 months after CE, indicating that 4αOH-CHO is also one of the biological activity markers of CYP3A4 and suggesting the regulation of 4αOH-CHO-CYP3A4 as the future direction for further research.
Previous studies have confirmed that coronary artery plaque imaging (Atherosclerosis T1-weighted Characterization, CATCH) can quickly and effectively determine the degree of long-term restenosis and the nature of atherosclerotic plaque in vitro. Long-term dynamic monitoring is of great significance, and CATCH is a new type of MRI technology. Our previous study used CATCH to effectively monitor the degree of restenosis and the nature of new AS plaques. Our results were comparable to traditional HE staining, and compared with coronary CTA, they were more accurate [2]. At the same time, combined with the medical imaging analysis based on NOMA technology used in previous studies [3], it could timely identify the tiny new AS plaques at the exfoliation site and, at the same time, calculate the coronary artery according to the maximum area of AS/coronary artery lumen area. The arterial stenosis rate improved the precision and reliability of the research results and provided an effective and novel technical method for observing the degree of restenosis in clinical trials.
This study has some limitations. First, the sample size was not big enough, which may affect the weight of the results. Second, the research and impact of 4αOH-CHO on other pathways disrupting cholesterol homeostasis still need to be further elucidated. Finally, the degree of interference also needs to be further refined.
In conclusion, this is the first study that demonstrated the effect of oxidized cholesterol 4αOH-CHO on the progression of AS and long-term restenosis after CE surgery by lipidomics analysis of CE specimens, finding that high expression of 4αOH-CHO could cause AS progression and thus affect the long-term patency rate of CE. Identifying 4αOH-CHO as a potentially biological activity marker of CYP3A4 may provide a new target for anti-AS therapy after CE, as well as provide a new idea for further elucidation of the mechanisms underlying AS. In this study, CATCH+NOMA's new MRI coronary plaque imaging + power domain non-orthogonal multiple access 5G integrated framework technology was used as a detection method after CE, thus providing a more advanced imaging analysis for AS plaques. An accurate acquisition and analysis method can be used as a new imaging method for the dynamic study of AS restenosis after CE.
Author contributions
CL and CF contributed to the conception, data collection, analysis, and drafted the manuscript. CL, CF, and GXL contributed to the analysis. XYX, XF, and BFL contributed to the data collection. YGL and XWC contributed to the conception and writing editing. BSZ contributed to the conception, design, acquisition of work, and critically revised the manuscript. All authors approved the submitted version.
Source of Financial Support
Natural Science Foundation of China (No. 82060082).
References
- Wang Chuan, Li Tongxun, Zhang Fan, et al. (2020) Preliminary study of endarterectomy combined with electrocautery in the treatment of diffuse coronary lesions. Chinese Journal of Thoracic and Cardiovascular Surgery 36: 83-88.
- Liu W, Xie Y, Wang C, Du Y, Nguyen C, et al. (2018) Atherosclerosis T1-weighted characterization (CATCH): evaluation of the accuracy for identifying intraplaque hemorrhage with histological validation in carotid and coronary artery specimens. J Cardiovasc Magn Reason 20: 27. [Crossref]
- Liu X, Liu Y, Yang Z (2022) Integrated 3C in NOMA-enabled Remote-E-Health Systems. IEEE Wireless Communications.
- Shui G, Cheong W F, Jappar IA (2011) Derivatization‐independent cholesterol analysis in crude lipid extracts by liquid chromatography/mass spectrometry: applications to a rabbit model for atherosclerosis. Journal of Chromatography A 1218: 4357‐4365.
- Li Yihua, Du Yu, Hong Bin (2020) Research progress on the regulation of low-density lipoprotein receptor expression. China Medical Biotechnology 15: 615-622.
- Liao Xinhui, Sun Hailing (2019) Effects of different statins on coronary heart disease and their effects on glucose and lipid metabolism and cardiac function. Medical Diet and Health 17: 46.
- Tiemuerniyazi X, Yan H, Song Y, Nan Y, Xu F, et al. (2021) Mid-term outcomes of coronary endarterectomy combined with coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 32: 188-195. [Crossref]
- Qiu Zhibing, Liu Yafeng, Jiang Yingshuo (2021) Short-term and mid-term effects of coronary endarterectomy combined with bypass grafting in the treatment of diffuse coronary stenosis. Chinese Journal of Surgery 59: 149-153.
- Wang Chuan, Gu Chengxiong (2016) Research progress in the application of coronary endarterectomy. Chinese Journal of Thoracic and Cardiovascular Surgery 32: 695-698.
- Nayor M, Brown KJ, Vasan RS (2021) The Molecular Basis of Predicting Atherosclerotic Cardiovascular Disease Risk. Circ Res 128: 287-303. [Crossref]
- Guo Mingqiu, Yin Xiaojie, Diao Dianyan (2021) The relationship between lipid metabolism and coronary atherosclerotic lesions. Chinese Journal of Arteriosclerosis 29: 149-155.
- Nitta SI, Hashimoto M, Kazuki Y, Takehara S, Suzuki H, et al. (2018) Evaluation of 4β-Hydroxycholesterol and 25-Hydroxycholesterol as Endogenous Biomarkers of CYP3A4: Study with CYP3A-Humanized Mice. AAPS J 20: 61. [Crossref]
- Aubry AF, Dean B, Diczfalusy U (2016) Recommendations on the Development of a Bioanalytical Assay for 4β-Hydroxycholesterol, an Emerging Endogenous Biomarker of CYP3A Activity. AAPS J 18: 1056 -1066.
- Huang MQ, Lin W, Wang W, Zhang W, Lin ZJ, et al. (2014) Quantitation of P450 3A4 endogenous biomarker - 4β-hydroxycholesterol - in human plasma using LC/ESI-MS/MS. Biomed Chromatogr 28: 794-801. doi: 10.1002/bmc.3131. [Crossref]




