This study characterizes the high-fat diet (HFD)-fed rats as a model for ‘pre-diabetes’ or ‘impaired glucose tolerance’ showing clinical presentation and pathophysiology of natural history of pre-diabetes in human. Young Wistar rats (40-50 g) were fed a HFD (45% energy from fat) or a normal diet for 6 month. Body weight gain was faster in 0-2 months, slower body weight gain in 2-4 months and slowest weight gain in 4-6 months. Blood glucose increased after 1 month on HFD feeding and remained elevated at a rate of ~5 mg/dl throughout the 6-monthstudy period. Serum insulin, insulin resistance and insulin-to-glucose ratio were increased progressively in a time-dependent manner. Progressive changes in glucose uptake and GLUT-4gene expression were also observed during 6-months of HFD feeding. The insulin immunohistochemical data showed hypertrophy of pancreatic islets in the pre-diabetic rats. We conclude that the HFD-fed rat model is a ‘humanized’ model for prediabetes, which may be used for studies on pathophysiology and development of new drug.
humanized rat model, impaired glucose tolerance, prediabetes, high fat diet, Glut4 mRNA, insulin immunohistochemistry
Diabetes is a chronic disease and for the most part a silent hidden disease. All diabetic subjects gone through a process called ‘pre-diabetes’ or ‘impaired glucose tolerance’ a condition in which the blood glucose is lower than the diabetes but higher than normal (fasting plasma glucose: 100 - <126 mg/dl; 2-h glucose: 140- < 200 mg/dl) [1]. It is estimated that up to 70% of people with pre-diabetes may develop type 2 diabetes during their lifetimes[2]. Obesity is one of the major risk factor that promotes the progression of pre-diabetes to type 2 diabetes, although genetic predisposition exists.
Insulin resistance and impaired insulin secretion in pre-diabetes is responsible for changes in fasting and postprandial blood glucose. However, the cellular and molecular mechanism(s) underlying these basic defects is not clear yet. Therefore, insulin resistance and impaired insulin secretion needs reliable and clinically relevant experimental models. Most animal models do not fulfill such requirements, since they have little relevance for human diabetes or on chemical destruction of β-cells, which is also of less clinical relevance.
A number of animal models have been reported and extensively used for diabetes research [3-7]. Explore the pathogenesis and for drug research. However, very few models were found for prediabetic model [8-12]. Moreover, the pathogenesis of pre-diabetes in animal models is most likely not similar to the pathogenesis of in human. Most interestingly, the cellular and molecular mechanism(s) is almost completely lacking in the pre-diabetes animal models. Here, we have developed and characterized a HFD-fed rat model for prediabetes that showed clinical presentation and pathophysiology of natural history of pre-diabetes that was found in human. Four (4) months HFD-feeding to young Wistar rats to developed insulin resistance and 6 months HFD feeding developed pre-diabetes in young Wistar rats. Progressive changes in glucose uptake and GLUT-4 gene expression were observed in this prediabetes model rats.
Animals and experimental design
Young male Wistar rats weighing (40-50 g) were used in this study. They were maintained at standard environmental conditions that is temperature 25 ± 2°C, relative humidity 50-55% and 12/12 hours light & dark cycles. After one week of acclimatization period, rats was fed with HFD (Rodent diet # D12451, Research Diet, Inc., New Brunswick) enriched with 45% fat that was fed on normal standardized diet without additional fat. The rats were fed HFD continuously for 6 months periods. Body weight, fasting and 2-h blood glucose was monitored every month. At 0, 2, 4 and 6 months blood samples were collected from fasting anesthetized rats for the measurement of serum insulin, total cholesterol, HDL-cholesterol and triglyceride level. Additionally, epididymal fat pads were rapidly isolated. After isolation, half of the tissue was rapidly frozen in liquid nitrogen and kept at -80°C until mRNA analysis. The other half of the tissue was rapidly washed with warm phosphate buffer pH 7.4 and immediately put for the isolation of adipocytes. At the end of experimental periods, the rats were sacrificed and a portion of the pancreas from the same anatomical location (splenic) from each rat was taken and fixed in 10% buffered formalin and processed for immunohistochemical analysis. Insulin and glucagon immunostaining as well as morphometry was done as described earlier [13,14]. Serum was separated from the collected blood samples for the determination of biochemical parameters. All animals were treated according to the guidelines for care and use of laboratory animals with the approval of Institutional Ethics Committee of the ICCBS, University of Karachi, Pakistan.
Serum parameters
Fasting serum glucose was measured by the glucose oxidase method (Randox Laboratories, Antrim, UK) and fasting serum insulin was measured using ultrasensitive rat insulin ELISA kit (Crystal Chem, Inc., Downers Grove, IL, USA). Serum triglyceride was measured by the enzymatic colorimetric method and serum total cholesterol and HDL-cholesterol was measured by the cholesterol oxidase/peroxidase method (Randox Laboratories).
Total RNA isolation and cDNA synthesis
Total RNA was isolated from the frozen part of the adipose tissues by using TRIAzol reagent according to the manufacturer’s instruction (Invitrogen, Waltham, Massachusetts, USA). Total RNA from 5-7 rats in each group was pooled and aliquots were subjected to further analysis. The concentration and purity of RNA was determined by using nanodrop. cDNA was synthesized from 5 µg of RNA using Revert Aid First strand cDNA synthesis Kit (Thermo Scientific, MA, USA).
Quantitative reverse transcriptase PCR (qPCR)
Real-time PCR instrument (Stratagene Mx3000p, SC, USA) were used for this study. Each PCR reaction of 20 µl comprised of 10 µl of 2X SYBR green master mix (Fermentas, Burlington, Canada),), 2.0 µl of template cDNA, 2.0 µl of GLUT-4 primer pair detailed below and 6.0 µl of nuclease free water. The primer sequences were: TGAGCCGCGACTGTGATG (sense) and GTCTCGGTGACAAAGTCGAAGTT (anti-sense) for GLUT-4 and GTCCGAGTCACCGCCTGCCG (sense) and CTCGGCTGGCGACGCAAAAG (anti-sense) forGlyceraldehyde-3-phosphate dehydrogenase (GAPDH). The genes were amplified using 30 cycles at 95°C for 30 s, 60°C for 30 s and 72°C for 30 s, followed by 5 min final extension at 72°C. GAPDH was amplified as housekeeping gene and used in the normalization of the threshold number of cycles (Ct) of GLUT-4 gene in real-time quantification, where Ct means the number of PCR cycle at which the measurable amount of fluorescence was produced by the amplified sample product. The method used to calculate the difference in GLUT-4 gene expression in relation with housekeeping gene involves the 2-∆∆Ct formula. The data obtained from this method tells us about fold change increase or decrease in the treated samples in comparison with control. Each sample was experimentally analyzed in triplicate. To make sure about the specificity of amplified products, melting curve for each individual sample was obtained at the end of amplification.
Other methods
Adipocytes isolation and 2-deoxy D-glucose (2DG) uptake assay was performed as described previously (Sohail and Hafizur, 2014). HOMA-IR index was calculated from fasting glucose and fasting serum insulin by using homeostasis model assessment (HOMA) calculator (version 2), where HOMA-IR indicates insulin resistance index.
Statistical analysis
All of the data are presented as means ± SEM from 12-17 rats/group. The statistical analyses were performed using SPSS for Windows (SPSS Inc., Chicago, IL, USA). Data were analyzedusing paired and un-paired t-test, as appropriate. Statistical significance is indicated by P < 0.05.
Changes of body weight and food intake of HFD-fed rats
In our HFD-fed experimental series of animals, HFD was introduced at weaning age of Wistar rats to half of the rats (n = 22), while the other half was maintained on the normal diet (n = 22). At this age, body weight was 47-52 g in both rats groups (Figure 1A). During the first week after introduction of HFD, body weight increased significantly (p<0.001) in the HFD–fed group (58.2 ± 7.8 g) compared to the normal diet-fed group (21.2 ± 4.2 g). The weight gain continued thereafter to be progressively higher in HFD–fed group (Figure 1A). The growth curves showed, however, similar patterns in the two groups, with a larger body weight gain over the first 8 weeks, followed by a slower weight gain during the subsequent 9-16 weeks. The growth was linear in both groups upto8 weeks, as illustrated in (Figure 1A). The growth rate in normal diet-fedrats during the first 8 weeks was 21.25 ± 5.06 g/week and this was increased to 34.37 ± 13.38 g/week (p<0.001) in HFD-fed rats, i.e., the weight gain was augmented by 60.0 ± 22.4 % by the HFD (p<0.001). The growth rate during the second phase, i.e., from week 9-16 was 6.1 ± 3.9 g/week in normal diet-fed rats versus 11.3 ± 5.8 g/week in HFD–fed rats (p<0.001); hence, the augmented growth rate was 42.7 ± 22.9 % during this phase (P<0.001). In 3rd phase (17-24) weeks, growth rate was almost similar in control (7.2 ± 2.5 g) and HDF-fed (6.5 ± 3.5 g) groups.
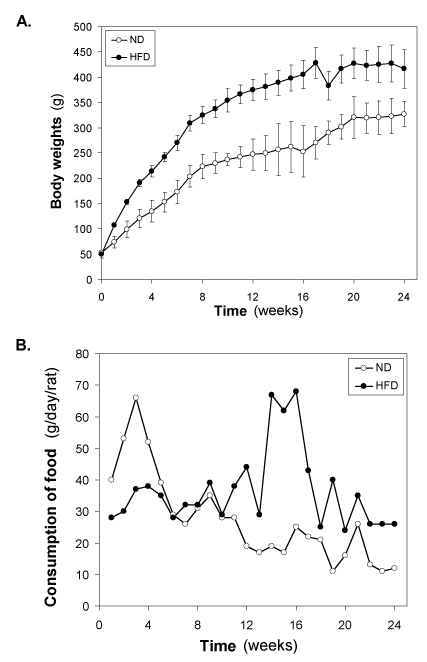
Figure 1. Changes of (A) body weight (g) and (B) consumption of food (g/day/rat) of the HFD- fed rats. Values are mean ±S.E.M. for 9-11 rats per group. ND, normal diet; HFD, high fat diet.
The food intake was initially lowered in HFD–fed groups compared with normal diet-fed group, however, increased from 7 weeks onwards in HFD-fed group compared with normal diet- fed rats throughout the study period (Figure 1B). After 7 weeks of HFD feeding, the difference between the two groups being stable.
Changes of fasting blood glucose and 2-h blood glucose of HFD-fed rats
Monthly samples were collected from fasting rats for measurements of plasma levels of glucose and insulin. At the start of the study, i.e., weaning age of rats, basal glucose was 77 – 82 mg/dl both the groups. There was little change of fasting blood glucose in the ND-fed groups, however, almost a linear increase in fasting blood glucose was observed in HFD-fed groups. The value 103 ± 2.4 mg/dl crossed at 4-month and reached 108 ± 3.9 mg/dl at 6-month (Figure 2A). No significance increase of blood glucose was observed after 6-months of HFD feeding (data not shown).
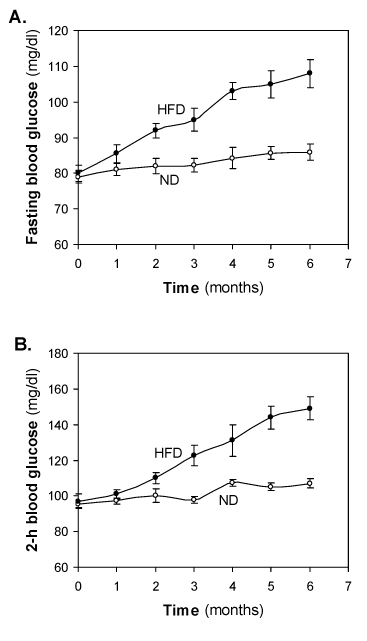
Figure 2. Effect of the HFD on (A) fasting blood glucose (mg/dl) and (B) 2 hour blood glucose (mg/dl). Values are mean ±S.E.M. for 9-11 rats per group. ND, normal diet; HFD, high fat diet.
At the start of the study 2-h blood glucose was 95.0 ± 0.1 and there was little change was observed in the ND-fed groups. In HFD-fed group, initial 1-2 months no significant glucose intolerance was observed. Significant glucose intolerance was observed at 3 month of HFD feeding. Glucose intolerance was increased in a time dependent manner. Crossed IGT cut-off value at significant IGT however, almost a linear increase in fasting blood glucose was observed in HFD fed groups. No mortality was observed during 6 month HFD feeding and success rate was of pre-diabetes was 00% in the HFD-fed rats.
Changes of serum insulin, HOMA-IR and insulin-to-glucose ratio of HFD-fed rats
The concentration of insulin in the HFD fed rats also increased gradually (Figure 3). After 2 months of HFD, the concentration of insulin was significantly (p<0.05) different to that of same time normal diet group. At 4th month, the difference between control and HFD groups increased very much >3-fold higher concentration of insulin in HFD fed group. At 6 month, the level of insulin decreased significantly (p<0.01) to that of 4 month HFD group’s level. The pattern of reduction of insulin levels in the HFD-fed group at 6 months suggests a progressive worsening of insulin function during HFD feeding. This is illustrated in (Figure 3B-3C), where the increase in HOMA- IR and insulin-to-glucose ratio levels in HFD-fed over insulin in normal diet-fed rats at each time point. It is seen that insulin-to-glucose ratio, which indirectly estimates augmented insulin resistance in HFD-fed rats, is linearly increased from 0-4 months, however, decreased at 6 months compared to 4th month value (Figure 3A-3C).
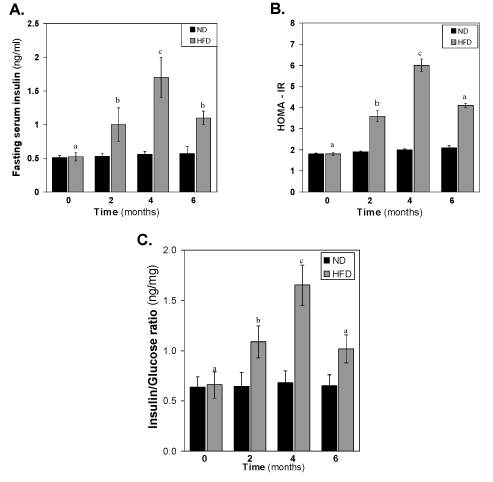
Figure 3. Effect of the HFD on (A) fasting serum insulin, (B) HOMA-IR and (C) Insulin-to-glucose ratio. Values are mean ±S.E.M. for 9-11 rats per group. Bars that do not share a common notation are significantly different at P<0.05. ND, normal diet; HFD, high fat diet.
The immunohistochemical analysis of pancreatic sections for insulin and glucagon showed increased islet size in HFD rat islets as compared to the normal diet rat islets (Figure 4). Islet size was almost double in pre-diabetic rats and intense insulin staining was observed in HFD rat islets compared to the normal diet rat islets.
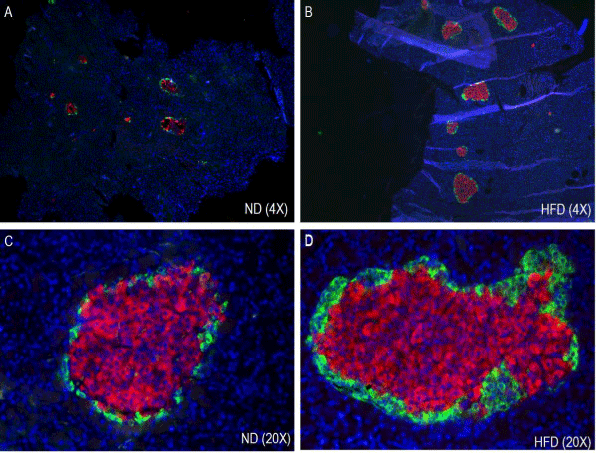
Figure 4. Effect of HFD on Immunohistochemistry and morphometry of pancreatic islets (α- and β-cells). ND, normal diet (A & C); HFD, high fat diet (B & D).
Changes of serum total cholesterol, HDL-cholesterol and triglyceride of HFD-fed rats
Figure 5 shows the effect of HFD feeding on total cholesterol, HDL-cholesterol and triglyceride levels. HFD feeding to Wistar rats did not show any significant change in the serum total cholesterol and HDL-cholesterol during the 6 months HFD feeding periods. However, the serum triglyceride of the HFD rats was increased significantly (p<0.01) compared to the normal diet group.
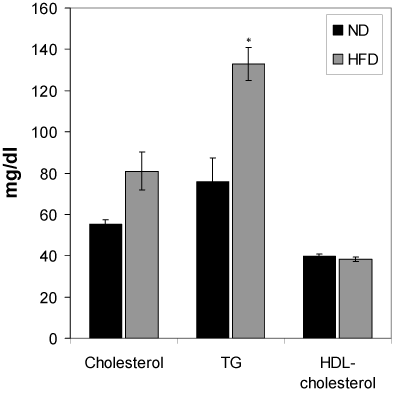
Figure 5. Effect of the HFD on cholesterol, triglycerides and HDL-cholesterol. Values are mean ±S.E.M. for 9-11 rats per group. *P<0.01 as compared to normal diet fed rats. ND, normal diet; HFD, high fat diet.
Changes of 2-deoxy-D-glucose (2DG) uptake in isolated adipocytes from HFD-fed rats
The uptake of 2DG by the adipocytes isolated from normal diet fed, 2-, 4-, and -6 months HFD fed groups were measured. The comparison was done in relative terms by taking the basal control as 100%. The basal and insulin stimulated 2DG uptake by the control rat’s adipocytes was significantly higher than all other groups (Fig. 6). However, the insulin stimulated 2DG uptake was significantly decreased in HFD 2-3 (P<0.05) and HFD 4-6 (p<0.01) groups.
Changes of GLUT-4 mRNA expression in isolated adipocytes from HFD-fed rats
As shown in the Figure 7, the total adipocytes content of Glut4 mRNA in all the HFD fed groups were decreased significantly (p<0.05) compared to the mRNA isolated from the normal diet fed group. The adipocytes content of GLUT-4 mRNA in adipose tissue remains 78 ± 2.9 % in the 2- month’s HFD-fed group to that of control. These GLUT-4 mRNA content further decreased drastically during the next 4 months HFD-fed period. This was the same period when the rats develop hyperglycemic condition. In the next 3 months, the expression level of GLUT-4 gene decreased further with the higher rate as compared to the initial 3-months decrease.
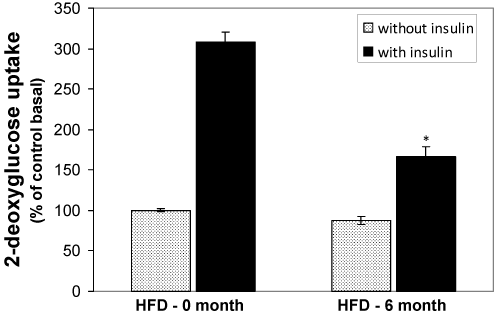
Figure 6. Effect of the HFD on insulin stimulated (black bars) and basal (grey bars) 2DG uptake in isolated adipocytes from the HFD-fed rats. Epididymal fat pads of treated and untreated rats were used for the preparation of adipocytes. The adipocytes were incubated in the presence of [14C] 2DG and then the cellular presence of 14°C were measured by liquid scintillation counter. Values are mean ±S.E.M. for six rats per group. Bars that do not share a common notation are significantly different at P<0.05. ND, normal diet; HFD, high fat diet.
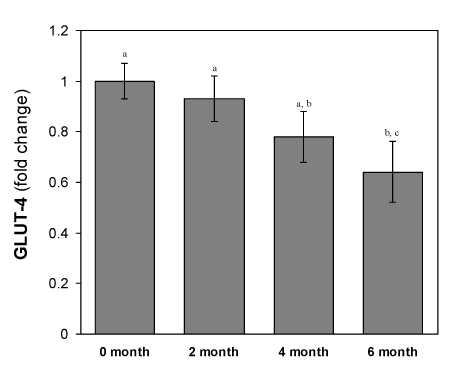
Figure 7. Changes in GLUT-4 mRNA levels of adipocytes of HFD-fed rats. Values are mean ±S.E.M. for 9-11 rats per group. RT-PCR results represent the mean ± SEM from a single experiment performed in triplicate. Bars that do not share a common notation are significantly different at P<0.05. ND, normal diet; HFD, high fat diet.
2021 Copyright OAT. All rights reserv
This study characterizes the HFD–fed rats as a ‘humanized’ model for pre-diabetes. We show here that HFD feeding to weaning rats results in increased body weight gain over time and stable hyperglycemia but a progressively increased hyperinsulinemia, indicating progressive worsening of insulin resistance. Furthermore, after 1 month on HFD, fasting blood glucose and serum insulin were significantly elevated and OGTT showed glucose intolerance in a time-dependent manner. The model thus shows two important mechanistic characteristics for pre-diabetes: insulin resistance and β-cell dysfunction. The growth curves of this 6-months study could be divided into 3 phases- first phase (0-8weeks) with more rapid growth, and a second phase (8-16 weeks) with slower growth and third phase (17-24 weeks) with slowest growth (Figure 1). Body weights and triglycerides, two predictors of obesity, also increased significantly in this HFD-fed group (Figures 1 and 5). At 4th month of HFD feeding, relatively high insulin concentration, less GLUT-4 mRNA expression and impaired glucose tolerance showed that the HFD-fed rats are insulin resistant at this stage. Beyond that, the curves of body weight gain and fasting glucose uplift relatively and reached to their maximum at 6th months (Figures 1A and 2A). At that time, fasting and 2-h blood glucose in the range of pre-diabetic range, increase serum insulin, HOMA-IR, insulin-to-glucose ration and decreased GLUT-4 mRNA expression (Figures 1-7) - all the data fulfill the conditions of pre- diabetic stage.
Pre-diabetic condition was further evaluated at cellular and molecular levels for further insights in the HFD-fed rat model. Increased islet size of prediabetes rats is rather hyperplasia of β-cells than the hypertrophy of individual β-cells per se. The increase in intensity of insulin in pre diabetes rat pancreatic islets is possibly because of increased demand of insulin by the body to achieve normoglycemia for which per cell synthesis of insulin is increased leading to intense staining. The body is still striving to achieve normoglycemia in the expanse of hyperinsulinemia. If this state of insulin resistance persists, there will be a continuous stimulus for the release of insulin and this vicious cycle continues posing a huge burden on β-cells. It is already described that increased workload on β-cell leads to ER stress that ultimately results in β-cell death and in long run, diabetes [15].
GLUT-4 is considered as major player of insulin stimulated glucose transport in adipose tissues. Insulin resistance is likely to be associated with the reduced transfer of GLUT-4containing vesicles but beside this mechanism(s) whether HFD also affecting the other mechanisms like the expression of GLUT-4 mRNA in adipocytes, we studied the Glut4 gene expression level in HFD-fed rats. The results showed that HFD also affected on the GLUT-4mRNA expression in a time-dependent manner (Figure 7).
In conclusion, we show here that the HFD feeding to weaning Wistar rats for 6-monthsdeveloped a rat model for pre-diabetes that showed clinical presentation and pathophysiology of natural history of pre-diabetes that was found in human. Therefore, this model may be more suitable and appropriate for both mechanistic studies and as a tool for developing novel therapeutic interventions.
This work was supported by a grant (No. 20-2177/NRPU/R&D/HEC/12(3654)) to Md. Hafizur Rahman from Higher Education Commission (HEC), Pakistan.
All authors declare no conflicts of interests in relation to this work.
- Li X, Lian FM, Guo D (2013) The rs1142345 in TPMT affects the therapeutic effect of traditional hypoglycemic herbs in pre-diabetes. Evidence-based Complementary and Alternat Med 327629.
- Nathan DM, Davidson MB, DeFronzo RA (2007) Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30: 753-759. [Crossref]
- Hemmings SJ, Spafford D (2000) Neonatal STZ model of type II diabetes mellitus in the Fischer 344 rat: characteristics and assessment of the status of the hepatic adrenergic receptors. Int J Biochem Cell Biol 32: 905-919. [Crossref]
- Tormo MA, Martinez IM, Romero de Tejada A, Gil-Exojo I, Campillo JE (2002) Morphological and enzymatic changes of the small intestine in an n0-STZ diabetes rat model. Exp Clin Endocrinol Diabetes 110: 119-123. [Crossref]
- Winzell MS, Ahren B (2004) The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53: S215-S219. [Crossref]
- Soares AF, Carvalho RA, Veiga FJ, Alves MG, Martins FO, et al.(2012) Restoration of direct pathway glycogen synthesis flux in the STZ-diabetes rat model by insulin administration. Am J Physiol Endocrinol Metab 303: E875-E885. [Crossref]
- Moore K, Ghatnekar G, Gourdie RG, Potts JD (2014) Impact of the controlled release of a connexin 43 peptide on corneal wound closure in an STZ model of type I diabetes. PLoS One 9: e86570.
- Tesch GH, Allen TJ (2007) Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 12: 261-266. [Crossref]
- Ellis CG, Goldman D, Hanson M, Stephenson AH, Milkovich S, et al. (2010) Defects in oxygen supply to skeletal muscle of prediabetic ZDF rats. Am J Physiol Heart Circ Physiol 298: H1661-H1670. [Crossref]
- Marsili A, Aguayo-Mazzucato C, Chen T, Kumar A, Chung M, et al. (2011) Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PLoS One 6: e20832. [Crossref]
- Wilson RD, Islam MS (2012) Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep 64: 129-139. [Crossref]
- Zuloaga KL, Krasnow SM, Zhu X, Zhang W, Jouihan SA, et al. (2014) Mechanism of protection by soluble epoxide hydrolase inhibition in type 2 diabetic stroke. PLoS One 9: e97529. [Crossref]
- Hafizur RM, Kabir N, Chishti S (2011) Modulation of pancreatic β-cells in neonatally streptozotocin-induced type 2 diabetic rats by the ethanolic extract of Momordica charantia fruit pulp. Nat Prod Res 25: 353-367. [Crossref]
- Hafizur RM, Kabir N, Chishti S (2012) Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function instreptozotocin-induced type 2 diabetic rats. Br J Nutr 108: 1586-1595. [Crossref]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, et al. (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50:752-763. [Crossref]







