Abstract
Background: Aspiration of gastric contents is common in the enterally-fed. Tinting enteral feedings with FD&C Blue No. 1 was thought to aid bedside detection of aspiration, yet its safety was never established. After several published reports had shown that dye absorption occurs in this setting and was associated with death, in 2002 we sought more information on clinician recognition of dye absorption from feedings, on patient risk factors, and on outcomes - with a goal of sharing data with the US Food and Drug Administration (FDA) in the interest of patient safety.
Methods: We completed a survey of 50 dietitians and 50 physicians at 100 hospitals in the United States in 2002-3 who cared for enterally fed patients. We evaluated the frequency of witnessed dye absorption between dietitians and physicians, patient comorbidities, presence of illnesses that increase gut permeability, outcome by level of discoloration, presence of acute renal failure, and analyzed any stored fluid samples.
Findings: Dietitians were more likely to encounter dye absorption than physicians (42% versus 20%, p = .017). Available data for 17 cases revealed most patients were critically ill and had conditions associated with increased gut permeability. None of these cases had been reported to the FDA. Blue skin discoloration was significantly associated with death (P < .01). In 2002 we facilitated reporting of all cases to the FDA using the MedWatch system. In 2003 the FDA issued a Public Health Advisory referencing these and other reports.
Conclusions: Absorption of FD&C Blue No. 1 from feedings was a serious and underreported complication. This approach highlights the design of a simple patient safety project, the underreporting of sporadic hospital complications, and the utility of surveying hospital dietitians for enteral feeding complications.
Key words
FD&C Blue No. 1, enteral nutrition, sepsis, intestinal permeability, aspiration, patient safety
Abbreviations
FDA: Food and Drug Administration; ICU: Intensive care unit
Introduction
The history of medicine provides many examples of practices being adopted before their efficacy and safety is established. One such practice was the tinting of enteral feedings with blue food dyes. Aspiration of oropharyngeal or gastric contents into the trachea occurs frequently in hospitalized patients. Patients receiving enteral feeding during mechanical ventilation are at particular risk [1]. As aspiration may cause nosocomial pneumonia, bedside methods to detect aspiration are desirable. The tinting of enteral feedings with blue dyes, usually FD&C Blue No. 1, was one method used for this purpose for decades (Figure 1) [2]. While prospective clinical studies had found the blue dye method to have a low sensitivity for aspiration [3-5], its ease of use, low cost, and presumed safety led to its popularity, particularly in United States intensive care units where up to 86% of nurses in 1999 reported using dye routinely [6].
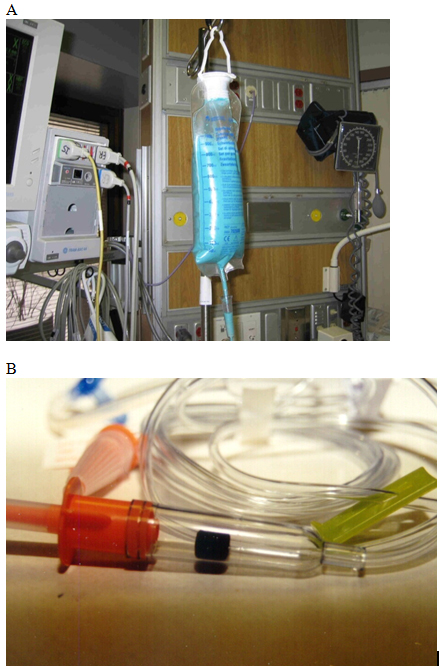
Figure 1. Methods of Tinting Enteral Feedings with FD&C Blue No. 1 Food Dye. Example of A) enteral feedings tinted with FD&C Blue No. 1 from a multiuse bottle, and B) the drip-chamber dye pellet tubing system also used at the time of the survey for FD&C Blue No. 1 tinting of enteral feedings.
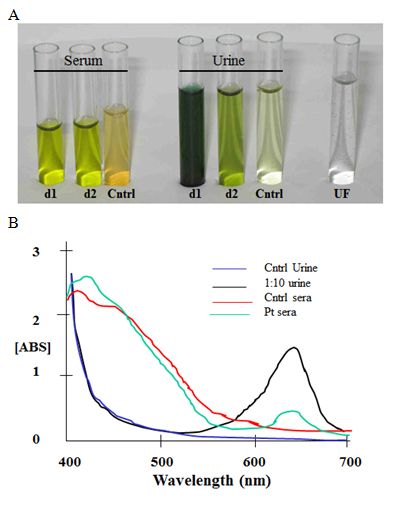
Figure 2. Blood and urine samples from an FD&C Blue No.1 Absorption Case. A man with sepsis who developed green urine, serum, and skin while receiving enteral feedings tinted with FD&C Blue No.1 (patient 13, Table 2). A) Green-tinted patient serum and urine from days 1 and 2 of discoloration (d1 and d2) alongside samples from a healthy subject (Cntrl). Continuous dialysis on day 1 was unsuccessful for dye removal; ultrafiltrate (UF) was clear and dye was undetectable. B) FD&C Blue No.1 content shown by spectroscopy at its absorption peak at 629 nm (ABS, arbitrary units) and compared to a standard curve of this dye. In these samples dye was quantified at 10 ug/ml in day 1 serum, and 100 ug/ml in day 2 urine.
Case reports of blue dye absorption from enteral feedings in 2000 raised concerns that this method was not as safe as originally thought [2,7-11], but the practice continued in many hospitals. The large amounts of dye administered, the propensity of hospitalized patients to have illnesses that increase gut permeability [12], and the inhibitory properties of FD&C Blue No. 1 on mitochondrial respiration suggested plausible mechanisms for both dye absorption and dye toxicity - particularly in the critically-ill [13,14]. However, FD&C Blue No. 1 absorption from enteral feedings had appeared uncommon with only 11 published reports (including professional meeting abstracts) by 2000 [15]. In 2002, despite such publications we continued to receive feedback at international nutrition meetings that practitioners had encountered additional cases of food dye absorption in their patients and that the practice continued to be embraced in their hospitals. We surmised that the sporadic nature of reports and confusion on how to report adverse events related to food additives led to a lack of pressure on government regulatory agencies (such as the US Food and Drug Administration, FDA) to examine and/or regulate the practice.
We hypothesized that dye absorption cases were under reported, that dye absorption was highly associated with illnesses that increase gut permeability, and that blue skin discoloration (indicating higher dye levels in blood) would be associated with death. In the interest of improving patient safety, in 2002-3 we conducted a national survey of dietitians and intensivists in order to test these hypotheses, with a linked goal of assisting respondents to report any cases to the US FDA using the Medwatch system. We present that data herein. In 2003 we felt our goals for the survey had been achieved as we reported these cases to the FDA which was soon followed by a formal review and their issue of a public health advisory on the practice, so we did not pursue publication of these data. However, in the current era where patient safety permeates hospital care, this survey provides a useful example of effective patient safety research of interest for its design, the data it captured, how it leveraged the participation of hospital dietitians, its delineation of patient characteristics associated with risk and outcome, and its use of the data to push a national regulatory review of a non-evidence-based practice.
Methods
Survey
A convenience sample of United States physicians and hospital-based dietitians were surveyed in 2002-3 by E-mail, telephone, or in person (at medical conferences) until 50 responses were obtained in each group. All respondents practiced at different adult hospitals in the USA, so these data represent data from 100 hospitals. 60 physicians and 58 dietitians were contacted to get these responses. Dietitians were selected based on membership in the American Society of Parenteral and Enteral Nutrition or the American Dietetic Association. Physicians were selected based on practice in critical care medicine (including 3 surgeons) using membership lists of the American Thoracic Society and American College of Chest Physicians. Participants were asked: “Have you encountered a patient who developed skin or fluid discoloration while receiving FD&C Blue No. 1 food dye in enteral feedings?” For “yes” responses participants were asked to provide details including the type and duration of dye use in feedings, the primary admit diagnosis, the location of care (ward or intensive care unit), whether comorbidities that increase gut permeability were present (sepsis, cardiac bypass, burns, inflammatory bowel disease), whether drugs associated with blue or green urine or skin discoloration were prescribed (amiodarone, propofol), presence of acute renal failure requiring renal replacement therapy, presence of known gut perforation, whether hyperthermia (>105 ºF) was observed, the character of discoloration (green or blue, urine versus skin discoloration), the availability of stored fluid samples, patient outcome, autopsy results, and whether cases were reported to the FDA. We excluded providers who used methylene blue (not a food dye) or who did not use blue food dye to tint feeds, or who previously reported cases. The Medical College of Wisconsin Institutional Review Board approved the study.
Human sample analysis
Quantification of FD&C Blue No. 1 in fluid samples was by light spectroscopy using the absorption wavelength (lmax, 629 nm) of FD&C Blue No. 1 and a standard curve of FD&C Blue No. 1 diluted in control serum or urine, as reported [10].
Statistical analysis
Two-tailed Fisher’s exact testing was used to analyze differences between respondents having encountered a dye absorption case based on occupation (dietitian versus physician) and to assess mortality differences related to green versus blue skin coloration (mortality within 3 days of discoloration onset). Significance was defined as p < 0.05.
Results
Food dye absorption from enteral feedings was under-reported
From 50 subjects in each group, 21 dietitians and 10 physicians reported encountering one or more cases of body or fluid discoloration they attributed to FD&C Blue No.1 absorption from enteral feedings. Of these 31 cases, detailed information was provided by respondents in 17 cases (16 cases by dietitians, one case by a physician; Table 1). Thus, dietitians were significantly more likely to have observed a dye absorption case compared to physicians (Table 2, 42% versus 20%, p = .017). Discoloration was observed in skin, urine, and pleural fluid (in patients who had existing chest tubes). Forty-six percent of dietitians and 89% of physicians who responded practiced at academic institutions. None of these cases had been previously reported in the medical literature, to the FDA, or to other regulatory agencies. We facilitated reporting of all 17 cases to the FDA using the MedWatch system by assisting responding dietitians with form completion and submission.
Table 1. Characteristics of 17 Cases of FD&C Blue No. 1 Absorption from Enteral Feedings
Case |
State |
Year |
Age in Years (sex) |
Wardor ICU |
History |
Illnesses of Gut Permeability |
Nature of
Discoloration¥ |
Outcome¥ |
Dye Use
(days) |
Source
of Dye |
1 |
ME |
1991 |
72 (M) |
ICU |
CABG, septic shock, MV |
CABG, sepsis |
Green: skin |
Lived |
3 |
Bottle |
2 |
LA |
1992 |
55 (F) |
ICU |
cardiac ischemia |
none reported |
Green: skin |
Lived |
UK |
Bottle |
3 |
NY |
1992 |
70 (M) |
ward |
stroke |
none reported |
Green: skin |
Lived |
14 |
Bottle |
4 |
LA |
2002 |
55 (M) |
ICU |
bowel resection |
IBD |
Green: urine |
Lived |
UK |
Bottle |
5 |
MO |
1996 |
30 (M) |
ICU |
sepsis, trauma, MV |
sepsis, trauma |
Green: urine |
Lived |
8 |
Bottle |
6 |
CA |
2000 |
70 (F) |
ICU |
sepsis, MV |
sepsis |
Green: skin, serum |
Died |
10 |
DPS |
7 |
CA |
2000 |
70 (F) |
ICU |
sepsis |
sepsis |
Blue: skin |
Died**RfS |
UK |
DPS |
8 |
NC |
2000 |
55 (F) |
ICU |
sepsis |
sepsis |
Green: urine |
Lived |
UK |
Bottle |
9 |
MA |
2000 |
79 (F) |
ICU |
bowel perforation, MV |
sepsis‡‡ |
Green: urine |
Lived |
4 |
Bottle |
10 |
CA |
2000 |
65 (M) |
ICU |
sepsis, ARF |
sepsis |
Blue: skin, urine |
DiedRfS |
UK |
DPS |
11 |
OH |
2001 |
81 (M) |
ICU |
sepsis, gut perforation, ARF,MV |
sepsis‡‡ |
Green: skin, urine,
PF‡ |
Lived |
1 |
Bottle |
12 |
AZ |
2001 |
68 (F) |
ICU |
septic shock, MV |
sepsis |
Green: skin, urine, PF |
Died**RfS |
8 |
DPS |
13 |
WI |
2001 |
54 (M) |
ICU |
septic shock, MV, esophageal leak, J-tube in peritoneum |
IBD, sepsis‡‡ |
Green: skin, serum, urine, PF‡ §§ |
Lived |
4 |
DPS |
14 |
OK§ |
2001 |
32 (M) |
ICU |
septic shock, MV |
sepsis |
Blue: skin, urine |
Died*RfS |
2 |
DPS |
15 |
OK§ |
2001 |
76 (M) |
ICU |
mitral valve surgery, shock, sepsis, MV, ARF |
cardiac bypass,
sepsis |
Blue: skin‡ |
Died*RfS |
12 |
DPS |
16 |
FL§ |
2001 |
NI |
ICU |
sepsis |
sepsis |
Green: skin |
LivedRfS |
UK |
DPS |
17 |
FL§ |
2001 |
NI |
ICU |
septic shock |
sepsis |
Blue: skin |
DiedRfS |
UK |
DPS |
State designation is by US Postal Service codes; year is the year when the case occurred
Abbreviations: ARF, acute renal failure requiring hemodialysis; bottle, multiuse nonsterile bottle; CABG,
coronary artery bypass grafting; DPS, drip pellet blue dye administration system; F, female; IBD, inflammatory bowel disease; ICU, intensive care unit; M, male; MV, requiring mechanical ventilation; NI, not released by provider due to medicolegal concerns; PF, pleural fluid; RfS, refractory shock (defined as needing 2 or more vasopressors); UK, unknown
* death in setting of limitation or withdrawal of care
** autopsy performed, no gut perforation, but other results not released (no other autopsies were reported)
‡ received amiodarone; ‡‡ GI tract perforation; § cases occurred at the same hospital; §§ dye detected performed in fluid samples by light spectroscopy
¥ death was associated with blue skin discoloration versus green skin or urine discoloration (p = 0.014)
Food dye absorption from enteral feedings occurred mostly in intensive care unit patients with sepsis
Almost all cases occurred in an intensive care unit (ICU) during illnesses known to be associated with increased gut permeability (sepsis was near universal, followed by cardiac surgery and inflammatory bowel disease; Tables 2 and 3) [16,17]. Gut perforation was uncommon and discovered only at surgery (of 2 cases autopsied, neither had gut perforation); similarly renal replacement therapy was uncommon. Poorly quantifiable FD&C Blue No. 1 administration methods (multiuse bottles) and quantifiable dye administration methods (a drip-chamber dye pellet tubing system that delivers 10 mg dye/hr; Flexiflo Colormark Pump Set, Ross Product Division, Abbott Laboratories, Columbus, OH) were equally employed, but all deaths were reported with the dye pellet tubing system. All patients with blue skin discoloration died of refractory shock within 3 days of discoloration onset, and were more likely to die than patients with green skin discoloration or isolated urine discoloration (6 of 6 cases vs. 2 of 11 cases, respectfully, p = .002; Table 1). No gender predominance was apparent and age reflected typical adult ICU populations (age 61 ± 15 years, mean ± SD). Hyperthermia was not reported. Most patients were mechanically ventilated. Two patients had received amiodarone; none had received propofol. We identified one patient who had stored clinical samples (patient 13, Table 2); his serum was light green and urine was dark green, and FD&C Blue No. 1 was detected in all samples except dialysis ultrafiltrate (Figure 2).
Table 2. Responses to Survey – Provider Encounters with Discoloration in Patients Receiving FD&C Blue No. 1 in Enteral Feedings
Health Care Providers (N) |
Percent of Respondents who Encountered Discoloration
and Type of Discoloration (N) |
Did not Encounter Discoloration (N) |
Dietitians (50) |
42% (21)* |
58% (28) |
Blue Skin |
Green Skin |
Urine |
20% (10) |
24%(12) |
20% (9) |
Physicians (50) |
20% (10)** ‡ |
80% (40) ‡ |
Blue Skin |
Green Skin |
Urine |
0 |
6% (3) |
16% (8) |
Providers were all based at adult hospitals and were in the USA
58 dietitians (8 did not respond) and 60 physicians (10 did not respond) were surveyed
* 7 dietitians had encountered ³ 2 cases of discoloration; 2 of 10 dietitians who reported blue skin discoloration each encountered two cases
**4 physicians encountered ³ 2 cases of discoloration
‡ 5 responses that were “maybe” or “not sure” (all physicians) are listed as “No” responses; physicians were less likely to have encountered a dye absorption case (p = 0.017 for comparison of physicians versus dietitians)
Table 3. Characteristics of the 17 FD&C Blue No.1 Food Dye Absorption Cases from Enteral Feedings Reported to the US FDA
|
Yes |
No |
P value |
ICU Location % (N) |
94% (16) |
6% (1) |
<0 .001 |
Illness that Increases gut permeability present*
- Sepsis
- Cardiac bypass
- IBD
|
88% (15)
88% (15)
12% (2)
12% (2) |
12% (2) |
0.004 |
Gut perforation documented |
17% (3) |
82% (14) |
<0 .001 |
Need for Renal Replacement Therapy |
17% (3) |
82% (14) |
<0 .001 |
FD&C Blue No.1 addition method –reported mortality
- Bottle
- Pellet drip system
|
0% (0)
53% (9) |
47%(8)
0% (0) |
<0 .001 |
Mechanical Ventilation |
53% (9) |
47% (8) |
NS |
Hyperthermia |
0 |
100% (17) |
<0 .001 |
Analysis of data from cases in Table 1
Abbreviations: IBD, inflammatory bowel disease; NS, not significant
*some subjects had several of these conditions
** all deaths (but not all discoloration events) reported in this small case series
involved the dye pellet drip system
Food dye absorption from enteral feedings was not related to the dye source
Of the 17 subjects who we were able to obtain detailed data on (Table 2), 8 subjects received dye from multiuse bottles and 9 received dye from the drip-pellet system. The 7 deaths among these subjects occurred in those administered FD&C Blue No. 1 from the dye-pellet system, demonstrating that dye absorption was not an anomaly of excessive dye use from multiuse bottles. Two respondents indicated that at the time, all enteral feedings in their hospital were administered using the dye-pellet system by protocol, whether inside or outside an intensive care unit.
Discussion
By 2000 the blue dye enteral feed tinting method was entrenched in most U.S. intensive care units as a bedside monitor of aspiration [6]. As there had only been 11 published case reports of FD&C Blue No. 1 absorption by 2001 (including abstracts) its occurrence appeared to be uncommon [15]. However, our 2002-3 survey reported herein of enteral feeding providers indicated that FD&C Blue No. 1 absorption from enteral feedings was in fact a widely recognized but underreported adverse event. For perspective, at the time in the United States there were an estimated 2,144 hospital-based dietitians and 10,244 intensivists (1996 data); yet we surveyed only 50 of each [18,19]. None of the cases reported had previously been communicated to regulatory agencies. Thus dye absorption among critically-ill, enterally-fed patients was more common than previous reports suggested and was widely underreported. Almost all patients were in ICUs and had sepsis, an illness associated with increased gut permeability, suggesting that a permissive context was required for dye absorption.
That dietitians were more likely to have encountered dye absorption and to provide case details is not surprising, as dietitians care for proportionally more enterally-fed patients. While it is intuitive to suspect that administration of larger dye quantities for longer periods (creating a larger intestinal load) is a prerequisite for dye absorption [15], in our survey absorption of FD&C Blue No. 1 occurred in cases even after a few days and when a commercial dye-pellet tubing was used, designed to deliver 10 mg dye/hr (within FDA guidelines at the time for healthy subjects). In fact, the dye-pellet tubing was as commonly associated with dye absorption as the addition of dye from multidose bottles. Moreover, the 7 deaths among these 17 subjects occurred in those administered the dye-pellet system. Due to possible reporting and time bias (the drip pellet system was a more recently marketed device, and may have been used exclusively in some hospitals), we cannot ascribe a higher mortality to use of either system, and notably deaths have been reported with various dye addition methods.
That the 5 blue skin discoloration cases reported here and that 4 previously reported cases of blue skin discoloration associated with FD&C Blue No. 1 absorption all died shortly thereafter is concerning [2,7]. The only report of survival after blue skin discoloration was in a child [11]. The high mortality associated with blue skin discoloration is consistent with a mechanism where high tissue concentrations of absorbed dye inhibit mitochondrial respiration. FD&C Blue No. 1 is a triphenylmethane dye derived from coal tar with potent inhibitory effects on mitochondrial respiration in-vitro, decreasing oxygen uptake in liver mitochondria by 78% at 0.1 mg/ml and by 90% at 0.8 mg/ml (1 mM) [13,14]. These are concentrations at which FD&C Blue No. 1 will discolor serum green and blue, respectively [15]. The mechanism of this inhibition is consistent with inhibition of ADP translocation across the inner mitochondrial membrane by structural mimicry of the purine ring, a similar mechanism as the poison atractyloside [20]. In a child who died after absorbing FD&C Blue No. 1 from enteral feeds, blue skin and serum indicated a serum dye concentration near 1 mg/ml [2], and hyperthermia, refractory shock, and acidosis suggested in-vivo uncoupling of respiration. However, hyperthermia was not reported by our respondents. Interestingly, most reported deaths following FD&C Blue No. 1 absorption from enteral feedings, including in this survey, have occurred during sepsis. Mitochondrial dysfunction is a key feature of sepsis linked with poor outcomes [21], and further dysfunction due to absorbed dye may exacerbate these effects [22].
The potential for in-vivo toxicity of absorbed FD&C Blue No. 1 in humans is supported by animal studies where chronic subcutaneous injections of it were associated with dramatic increases in mortality [23,24]. Such unexpected toxic effects from parenteral dye in studies designed to assess carcinogenic potential were apparently deemed irrelevant to the safety of a food dye deemed nonabsorbable in healthy animals [25]. In 1982 the FDA recognized that existing studies were too small [26], and larger studies were commissioned in rodents that again suggested oral FD&C Blue No. 1 was safe [27]; yet further parenteral administration studies were not pursued. All animals were healthy in these studies, and thus would have normal gut permeability. Such work emphasizes that the safety of artificial food dyes, whether in enteral feedings or in foods, is predicated on their remaining nonabsorbable. The FDA never approved FD&C Blue No. 1 for use in enteral feeds except with a dye-pellet device, but FDA guidelines on acceptable ingestion in healthy adults of 12 mg/kg/day were extrapolated toward its use in enteral feeds [2,26].
Dye absorption in our survey occurred in diseases associated with increased gut permeability [12,28], mostly sepsis [15], but also with chronic illnesses like inflammatory bowel disease [29]. Of note, ICU admission itself has been associated with enhanced gut permeability [16]. Our findings combined with prior animal and human evidence suggest that the long-term safety of artificial food dyes in individuals with chronic illnesses that increase gut permeability (celiac sprue, atopic disease) should also be regarded as unestablished [16,23]. In healthy animals 90% of intravenously administered FD&C Blue No. 1 is excreted in bile, with only 10% excreted in urine [26,30]. Once absorbed from a leaky gut, FD&C this dye travels a classic enterohepatic cycle of biliary excretion and gut reabsorption where substantial liver exposure may occur before any discoloration is apparent [2]. Absorbed dye likely impairs function of the mitochondria-rich liver. Of relevance, marked blue-green liver discoloration was seen at autopsy in a patient who died after 14 days of FD&C Blue No. 1 in enteral feeds [15].
As our survey was not a randomized sampling of providers, we cannot extrapolate a national incidence of dye absorption cases then, nor can we rule out an ascertainment bias for clinicians more likely to have encountered dye absorption. Exclusion of gut perforation as a risk factor for dye absorption was not always possible due to a paucity of autopsies (gut perforation was identified in 3 cases at surgery, but was absent in the 2 autopsied cases), but it was not been reported in other published cases of dye absorption [2,7]. Other substances that can cause blue skin or green urine discoloration (amiodarone, propofol) cannot explain these cases given a minimal prevalence and/or the lack of absorption peaks for these drugs in assayed fluids in this and other reports [31-33].
Prospective studies had shown that the blue dye practice had a low sensitivity for aspiration detection, yet it was still embraced [3,34]. In 2002 consensus committee guidelines had recommended that the blue dye practice be abandoned based on poor sensitivity and potential for harm [35]. Yet the blue dye method continued to be utilized in American hospitals, with 48% of providers in a 2002 survey indicating their hospitals continued its use (though use had declined since 1999) [36]. At the same time, evidence-based measures shown to prevent aspiration remained underutilized [35,37]. Patient positioning with the torso elevated 30-45° in enterally-fed mechanically ventilated patients had been shown to decrease gastroesophageal reflux, aspiration, and nosocomial pneumonia in prospective studies published as far back as 1992 [1,37-39]. Nonrecumbent positioning was recommended for nosocomial pneumonia prevention by the Centers for Disease Control and the FDA [40], but it remained underutilized until promulgated over the ensuing decade by multiple safety-based organizations such as the Institute for Health Care Safety, the Leapfrog Group, and the National Advisory Forum. At our request, the 17 detailed cases identified in our survey were subsequently reported to the FDA by providers with our assistance, and were reviewed by the FDA preparatory to the drafting of their Public Health Advisory in September 2003 regarding potential hazards of this practice [40].
Conclusions
We found in 2002-3 that FD&C Blue No. 1 absorption was a well-recognized and underreported complication of enteral feeding in critically-ill patients who had common illnesses such as sepsis that increase gut permeability. Death in all patients with blue skin discoloration suggests that toxic properties of FD&C Blue No. 1 caused adverse effects in-vivo. The tinting of enteral feedings with FD&C Blue No. 1 was abandoned in the USA after a US FDA Health advisory in 2003 as it was an insensitive practice that was never evidence-based, and was potentially unsafe due to the ubiquitous illnesses that increase gut permeability and thus dye absorption. Our patient safety efforts with collecting, reporting, and analyzing data from this survey, as well as facilitating reports to the FDA, are a useful example for young clinicians interested in patient safety projects and provide a cautionary reminder of the potential downfalls of non-evidence based medical practices.
Acknowledgements
We thank the dietitians and physicians who participated in the survey, particularly those willing to provide details of their cases and report them to the FDA. The study is supported in part by National Institutes of Health grant HL03545 (JPM). No competing interests exist for either author.
References
- Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, et al. (1999) Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 354: 1851-8.
- Maloney JP, Ryan TA, Brasel KJ, Binion DG, Johnson DR, et al. (2002) Food dye use in enteral feedings: a review and a call for a moratorium. Nutr Clin Pract 17: 169-181. [Crossref]
- Potts RG, Zaroukian MH, Guerrero PA, Baker CD (1993) Comparison of blue dye visualization and glucose oxidase test strip methods for detecting pulmonary aspiration of enteral feedings in intubated adults. Chest 103: 117-121. [Crossref]
- Liu DW, McIntyre RW, Watters JM (1989) Pulmonary Aspiration in Critically Ill Patients receiving Enteral Feeding. Clin Investig Med 22: B119.
- Montejo-González JC, Pérez-Cardenas MD, Fernández-Hernández AI, Conde-Alonso MP (1994) Detecting pulmonary aspiration of enteral feeding in intubated patients. Chest 106: 1632-1633. [Crossref]
- Metheny NA, Aud MA, Wunderlich RJ (1999) A survey of bedside methods used to detect pulmonary aspiration of enteral formula in intubated tube-fed patients. Am J Crit Care 8: 160-167. [Crossref]
- Maloney JP, Halbower AC, Fouty BF, Fagan KA, Balasubramaniam V, et al. (2000) Systemic absorption of food dye in patients with sepsis. N Engl J Med 343: 1047-1048. [Crossref]
- Ehrig F, Waller S, 2021 Copyright OAT. All rights reservof 'green urine'. Nephrol Dial Transplant 14: 190-192. [Crossref]
- Czop M1, Herr DL (2002) Green skin discoloration associated with multiple organ failure. Crit Care Med 30: 598-601. [Crossref]
- Carpenito G, Kurtz I (2002) Green urine in a critically ill patient. Am J Kidney Dis 39: E20. [Crossref]
- Zillich AJ, Kuhn RJ, Petersen TJ (2000) Skin discoloration with blue food coloring. Ann Pharmacother 34: 868-870. [Crossref]
- Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, et al. (1998) Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med 158: 444-451. [Crossref]
- Reyes FG, Valim MF, Vercesi AE (1996) Effect of organic synthetic food colours on mitochondrial respiration. Food Addit Contam 13: 5-11. [Crossref]
- Noda K, Yoshimoto M, Hatano S, Watanabe T (1985) Effect of coal tar dyes on oxygen uptake in mitochondria isolated from rat liver. J Food Hyg Soc Japan 26: 203-207.
- Maloney JP, Ryan TA (2002) Detection of aspiration in enterally fed patients: a requiem for bedside monitors of aspiration. JPEN J Parenter Enteral Nutr 26: S34-41. [Crossref]
- Bjarnason I, MacPherson A, Hollander D (1995) Intestinal permeability: an overview. Gastroenterology 108: 1566-1581. [Crossref]
- Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, et al. (1996) Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA 275: 1007-12.
- Rogers D, Salary Survey Work Group (2003) Report on the ADA 2002 dietetics compensation and benefits survey. J Am Diet Assoc 103: 243-255. [Crossref]
- Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J (2000) Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA 284: 2762-70.
- Scherer B, Grebe K, Riccio P, Klingenberg M (1973) The new atractyloside type compound as a high affinity ligand to the adenine nucleotide carrier. FEBS Lett 31: 15-19. [Crossref]
- Brealey D, Brand M, Hargreaves I (2002) Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219-23.
- Clay AS, Behnia M, Brown KK (2001) Mitochondrial disease: a pulmonary and critical-care medicine perspective. Chest 120: 634-648. [Crossref]
- Hansen WH, Fitzhugh OG, Nelson AA, Davis KJ (1966) Chronic toxicity of two food colors, brilliant blue FCF and indigotine. Toxicol Appl Pharmacol 8: 29-36. [Crossref]
- Price PJ, Suk WA, Freeman AE, Lane WT, Peters RL, et al. (1978) In vitro and in vivo indications of the carcinogenicity and toxicity of food dyes. Int J Cancer 21: 361-367. [Crossref]
- Brown JP, Dorsky A, Enderlin FE, Hale RL, Wright VA, et al. (1980) Synthesis of 14C-labelled FD & C Blue No. 1 (Brilliant Blue FCF) and its intestinal absorption and metabolic fate in rats. Food Cosmet Toxicol 18: 1-5. [Crossref]
- Food and Drug Administration. FD&C Blue No. 1 (1982) Federal Register 47: 42563-66.
- Borzelleca JF, Depukat K, Hallagan JB (1990) Lifetime toxicity/carcinogenicity studies of FD & C Blue No. 1 (brilliant blue FCF) in rats and mice. Food Chem Toxicol 28: 221-234. [Crossref]
- O'Dwyer ST, Michie HR, Ziegler TR, Revhaug A, Smith RJ, et al. (1988) A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg 123: 1459-1464. [Crossref]
- Meddings JB (1997) Review article: Intestinal permeability in Crohn's disease. Aliment Pharmacol Ther 11: 47-53. [Crossref]
- Iga T, Awazu S, Nogami H (1971) Pharmacokinetic study of biliary excretion. II. Comparison of excretion behavior in triphenylmethane dyes. Chem Pharm Bull (Tokyo) 19: 273-281. [Crossref]
- Motsch J, Schmidt H, Bach A, Böttiger BW, Böhrer H (1994) Long-term sedation with propofol and green discolouration of the liver. Eur J Anaesthesiol 11: 499-502. [Crossref]
- Di Pietra AM, Cavrini V, Gatti R, Raggi MA (1988) Determination of amiodarone hydrochloride in pharmaceutical formulations by derivative UV spectrophotometry and high-performance liquid chromatography (HPLC). Pharm Res 5: 709-12.
- Court MH, Hay-Kraus BL, Hill DW, Kind AJ, Greenblatt DJ (1999) Propofol hydroxylation by dog liver microsomes: assay development and dog breed differences. Drug Metab Dispos 27: 1293-1299. [Crossref]
- Metheny NA, Dahms TE, Stewart BJ, Stone KS, Edwards SJ, et al. (2002) Efficacy of dye-stained enteral formula in detecting pulmonary aspiration. Chest 122: 276-281. [Crossref]
- McClave SA, DeMeo MT, DeLegge MH, DiSario JA, Heyland DK, et al. (2002) North American Summit on Aspiration in the Critically Ill Patient: consensus statement. JPEN J Parenter Enteral Nutr 26: S80-85. [Crossref]
- American Society of Parenteral and Enteral Nutrition. Accessed June 2003, http://www.nutritioncare.org/prevsurveys.html#bluedye.
- Cook DJ, Meade MO, Hand LE, McMullin JP (2002) Toward understanding evidence uptake: semirecumbency for pneumonia prevention. Crit Care Med 30: 1472-1477. [Crossref]
- Ibanez J, Penafiel A, Raurich JM, Marse P, Jorda R, et al. (1992) Gastroesophageal reflux in intubated patients receiving enteral nutrition: effect of supine and semirecumbent positions. J Parenter Enteral Nutr 16: 419-22.
- Torres A, Serra-Batlles J, Ros E, Piera C, Puig de la Bellacasa J (1992) Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Int Med 116: 540-43.
- http://www.fda.gov/forindustry/coloradditives/coloradditivesinspecificproducts/inmedicaldevices/ucm142395.htm.


