Background: Recently, our group reported that the acute rise in invasive systolic blood pressure (BP) during renal nerve stimulation (RNS) is a significant marker of the location of the renal nerves and suggest the importance of renal sympathetic activity in the mechanisms of hypertension.
Objective: The primary goal of this study evaluated if carotid sinus stimulation (CSS) and renal nerve stimulation (RNS) can provoke changes in the BP and HR.
Materials/Patients and Methods: This transversal study involved 18 uncontrolled hypertensive patients, complaining of pre-syncope. The study was piloted in agreement with the Helsinki declaration and approved by the ethics committee of our institution.
Results and discussion: Our study shows that an acute fall in invasive systolic BP and HR provoked by the CSS is countered by an increase in these parameters caused during RNS. These findings suggest the importance of the vagal tone offsetting the sympathetic activity as one of the pathways to suppress hypertension.
blood pressure, heart rate, carotid sinus stimulation, renal nerve stimulation, uncontrolled hypertension
Osteoarthritis (OA) is the leading cause of musculoskeletal morbidity in the elderly, the hands being one of the most
Extended electric stimulation of the carotid baroreceptors through its modulatory effect on afferent signaling is an attractive target for the treatment of uncontrolled hypertension. Baroreflex sensitivity is usually distressed in hypertension and be unsuccessful to keep blood pressure (BP) at proper levels through its fast-negative feedback loop, in which a raised BP reflexively origins the heart rate to fall and BP to drop. In an initial proof-of-concept study, BAT has been found to exert considerable BP-lowering properties using the CVRx Rheos System (CVRx, Minneapolis, MN) (DEBuT-HT [Device Based Therapy in Hypertension Trial) comprising implantable electrodes connected to a subcutaneously placed stimulator [1,2]. Implantation of the first-generation device was related to serious procedure-related adverse events, and the short-term battery life restricted its utility. Next-generation devices could essentially overawe these issues and implantation of the Barostim neo (CVRx) was associated with a significant BP decline at 3 and 6 months of follow-up [3]. A recent study in 28 patients [4] exposed further evidence of the positive effects reached by BAT in patients who continued hypertensive despite renal sympathetic denervation performed 5 months earlier. While the BAT device is typically initiated 2 to 4 weeks after surgical implantation to permit the site to heal, immediate activation of BAT in this specific situation created a quick, significant, and sustained reduction In BP. Even though a device that needs surgical placement of electrodes maybe impractical in an emergency condition, the concept of electrical stimulation of the baroreceptors as a means of accomplishing speedy and sustained BP reduction is fascinating and highlights the prospective of this methodology. The well-recognized association between augmented baroreceptors response and parasympathetic activation as an important contributor to the pathophysiology of hypertension led to the initiation of studies investigating the feasibility of a therapeutic intervention aimed at selectively targeting arterial baroreceptors located in the carotid sinus. Recently, our group reported that the acute rise in invasive systolic BP during renal nerve stimulation (RNS) is a significant marker of the location of the renal nerves and suggest the importance of renal sympathetic activity in the mechanisms of hypertension [5]. Our group believes that patients with uncontrolled hypertension submitted to an acute fall in invasive systolic BP and heart rate (HR) provoked by the coronary sinus stimulation (CSS) can be countered by an increase in these parameters caused during RNS.
This transversal study involved 18 uncontrolled hypertensive patients, complaining of pre-syncope. The study was piloted in agreement with the Helsinki declaration and approved by the ethics committee of our institution. All patients signed the informed consent term before inclusion. This study was conducted at the Hospital e Clínica São Gonçalo, Rio de Janeiro, Brazil. Patients were recruited from January to August 2016 from the Arrhythmias and Artificial Cardiac Pacing Service of the same hospital. Patients with the combination of the following criteria were consecutively enrolled: (i) mean 24-hour systolic ambulatory blood pressure measurements (ABPM) ≥130/≥80mmHg despite treatment with non-pharmacological measures and use of at least three antihypertensive drugs (including a diuretic) on maximally tolerated doses or confirmed intolerance to medications; (ii) CKD: glomerular filtration rate estimated by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, eGFR, [6] >60mL/min/1.73m2 without microalbuminuria; (iii) age from 18 to 80 years; (iv) complaining of pre-syncope symptoms; (v) negative tilt-table testing and normal carotid sinus compression; (vi) normal anatomy and function at rest and during stress assessed by the cardiac magnetic resonance and normal electrophysiological study (EPS); (vii) normal ultrasound Doppler of the carotid and vertebral arteries; (viii) able to read, understand and sign the informed consent form, and attend clinic visits and exams. The patients that presented any of the subsequent criteria were excluded: (i) pregnancy; (ii) valvular disease with significant adverse sequelae; (iii) unstable angina, myocardial infarction, transient ischemic attack or stroke within the 6 months before the procedure; (iv) renovascular abnormalities; (v) psychiatric disease; (vi) allergy to ionic contrast; (vii) the inability to be monitored clinically after the procedure; (viii) a known addiction to drugs or alcohol that affects the intellect; (ix) a serious health condition that, in the investigator opinion’s, may adversely affect the safety and/or efficacy of the participant or the study; (x) congestive heart failure presenting functional class II to IV symptoms according to New York Heart Association classification.
The 12 subjects underwent EPS. The primary goal of this study evaluated if carotid sinus stimulation (CSS) and renal nerve stimulation (RNS) can provoke changes in the BP and HR. The procedures were performed in the catheterization laboratory with direct visualization using fluoroscopy and radiopaque contrast. The patients were pretreated with diazepam or midazolam by an anesthesiologist. At the end of standard EPS, the femoral artery was punctured bilaterally, and a short 8F sheath (St. Jude Medical, St. Paul, Minnesota, USA) was placed into this vessel on both sides, unfractionated heparin was managed as an intravenous bolus, targeting an activated coagulation time of >250 s in the first 10 min. During the procedure, the target activated coagulation time ranged from 250 to 350 s, allowing introduce and position the quadripolar dirigible Livewire™ catheter (St. Jude Medical, St. Paul, Minnesota, USA) with a tip electrode of 2 mm, and other electrodes of 1 mm, alternetaly into the carotid arteries, as close as possible to the carotid sinus on both sides, under fluoroscopic guidance. To perform the RNS, an aortography, and selective renal arteriographies were obtained with an RDC catheter to visualize the aorta and renal arteries. Through the RDC catheter another conventional dirigible quadripolar catheter Livewire™ (St. Jude Medical, St. Paul, Minnesota, USA), with a tip electrode of 2 mm, and other electrodes of 1 mm was introduced into the renal artery, under fluoroscopic guidance and by the three-dimensional mapping system (EnSite Velocity; St. Jude Medical, St. Paul, Minnesota, USA) to anatomically construct the renal arteries and aorta and perform RNS in the selected sites. In both procedures, the unipolar stimulation was performed from the tip of the catheter to target the carotid sinus. The cycle length was set at 200 ms (300 ppm), pacing using the maximum-output voltage at 20 V, current of 8 mA, using a pulse amplitude of 2 ms, during 90 s. The EP-Tracer system (Schwarzer Cardiotek GmbH, Im Zukunftspark 3, 74076 Heilbronn, Germany) was used to record and monitor the cardiac rhythm and HR throughout the procedure. And the DX2021 monitor system (Dixtal Biomédica Indústria e Comércio LTDA., SP, Brazil) was used to register the changes in invasive systolic BP during the procedure. After the stimulation, we waited for the BP and HR to return to baseline values. The patients remained hospitalized in the ward for 24 h after the procedure.
The results are expressed as a mean and standard deviation for normally distributed data and as median with interquartile range otherwise. All statistical tests were two-sided. Comparisons between two-paired values were performed with the paired t-test in cases of a Gaussian distribution and by the Wilcoxon test otherwise. Comparisons between more than two-paired values were made by repeated-measures analysis of variance or by Kruskal–Wallis analysis of variance as appropriate, complemented by a post-hoc test. Categorical variables were compared with Fisher’s exact test. A P-value <0.05 was considered significant. All statistical analyses were performed using the program Graphpad Prism v 7.0 (Graphpad Software, La Jolla, CA, USA).
The general features of patients are listed in Table 1. According to Figure 1A, from the baseline values the right CSS provoked a decrease in the systolic BP of -31.0 ± 8.8 mmHg during stimulation (P<0.0001), the right RNS led to an increase in the systolic BP of +28.7 ± 8.3 mmHg during stimulation (P<0.0001), and the right CSS + RNS at the same time caused a non-expressive effect in the systolic BP of -0.3 ± 3.2 (P=0.9826). The Figure 1B shows that from the baseline values the left CSS provoked a decrease in the systolic BP of -27.1 ± 7.2 mmHg during stimulation (P<0.0001), the left RNS led to an increase in the systolic BP of +22.9 ± 7.8 mmHg during stimulation (P<0.0001), and the left CSS + RNS at the same time caused a non-expressive effect in the systolic BP of -0.2 ± 4.9 (P>0.9999). The comparison between right CSS vs. left CSS, right RNS vs. left RNS, and right CSS + right RNS vs. left CSS + left RNS was not significant (P=0.2443, P=0.0950, and P=0.9218, respectively). Regarding the HR, the Figure 2A shows that from the baseline values the right CSS provoked an HR decrease of -24.3 ± 2.9 bpm during stimulation (P<0.0001), the right RNS led to an HR increase of +25.8 ± 5.1 bpm during stimulation (P<0.0001), and the right CSS + RNS simultaneously caused a non-expressive effect in the HR of +0.8 ± 3.5 bpm (P=0.9866). The Figure 2B shows that from the baseline values the left CSS provoked a HR decrease of -12.3 ± 2.8 bpm during stimulation (P<0.0001), the left RNS led to an HR increase of +24.8 ± 4.2 bpm during stimulation (P<0.0001), and the left CSS + RNS at the same time caused a non-expressive effect in the HR of -0.3 ± 4.3 bpm (P>0.9999). The comparison between right CSS vs. left CSS was expressive (∆=12.0 ± 1.2 bpm, P<0.0001). However, the comparison between right RNS vs. left RNS, and right CSS + right RNS vs. left CSS + left RNS was not significant (P=0.6069 and P=0.5399, respectively).
Table 1. Baseline features
N |
12 |
Age, years |
56.7 ± 9.3 |
Body mass index, kg/m2 |
28.5 ± 3.0 |
Male gender (%) |
7 (58%) |
White ethnicity (%) |
8 (67%) |
Uncontrolled hypertension |
12 (100%) |
Type 2 Diabetes Mellitus (%) |
3 (25%) |
Coronary artery disease |
1 (8%) |
Normal carotid sinus compression |
12 (100%) |
Negative tilt-table test |
12 (100%) |
Normal electrophysiological study |
12 (100%) |
Normal cardiac magnetic resonance |
12 (100%) |
Mean 24-hour systolic/diastolic ABPM, mmHg |
147.1 ± 8.0/100.8 ± 4.6 |
Mean ISBP before right side stimulation, mmHg |
163.7 ± 9.5 |
Mean ISBP before left side stimulation, mmHg |
163.2 ± 7.7 |
Mean HR before right side stimulation, bpm |
78.4 ± 5.4 |
Mean HR before left side stimulation, bpm |
76.6 ± 6.3 |
Creatinine, mg/dL |
1.00 ± 0.22 |
eGFR, mL/min/1.73 m2 (CKD-EPI) |
83.5 ± 14.3 |
Albumin:creatine ratio mg/g |
13.0 ± 8.1 |
Antihypertensive agents |
|
ACEI/ARB |
12 (100%) |
Diuretics |
12 (100%) |
DHP Ca++ channel blockers |
12 (100%) |
β-blockers |
12 (100%) |
Spironolactone |
8 (67%) |
The values are presented as mean ± SD or %; ABPM, ambulatory blood pressure measurements; ACEI, receptor inhibitor of angiotensin converting enzyme; ARB, angiotensin receptor blocker; DHP, dihydropyridyne; eGFR, estimated glomerular filtration rate; HR, heart rate; ISBP, invasive systolic blood pressure; N, number of patients.
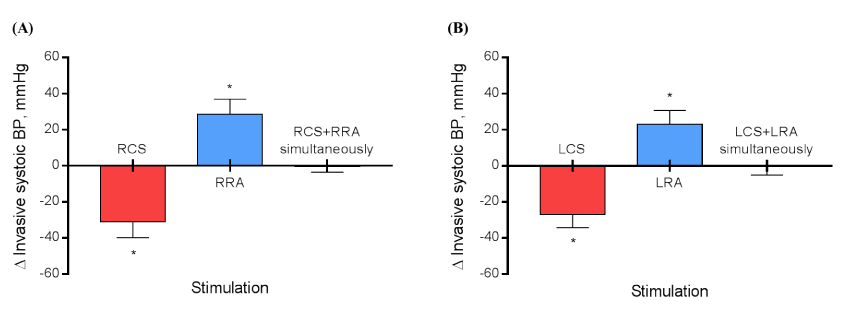
Figure 1. Variation (∆) in invasive blood pressure during the stimulation of right carotid sinus, right renal artery, and right carotid sinus + right renal artery simultaneously (A) and the left carotid sinus, left renal artery, and left carotid sinus + left renal artery simultaneously (B).*P<0.0001; BP, blood pressure; LCS, left carotid sinus; LRA, left renal artery; RCS, right carotid sinus; RRA, right renal artery; N=12.
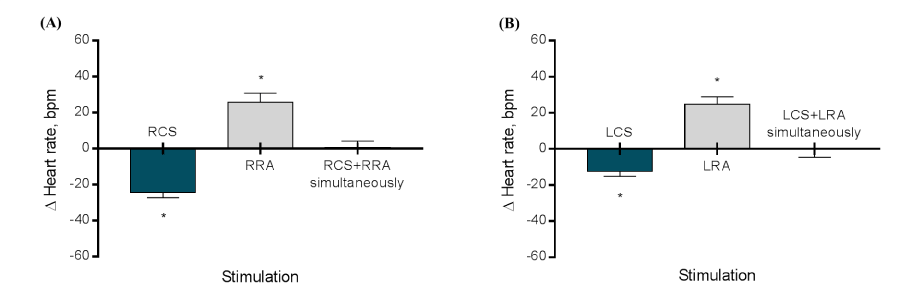
Figure 2. Variation (∆) in heart rate during the stimulation of right carotid sinus, right renal artery, and right carotid sinus + right renal artery simultaneously (A) and the left carotid sinus, left renal artery, and left carotid sinus + left renal artery simultaneously (B).*P<0.0001; HR, heart rate; LCS, left carotid sinus; LRA, left renal artery; RCS, right carotid sinus; RRA, right renal artery; N=12.
In conclusion, our study shows that an acute fall in invasive systolic BP and HR provoked by the CSS is countered by an increase in these parameters caused during RNS. These findings suggest the importance of the vagal tone offsetting the sympathetic activity as one of the pathways to suppress hypertension.
None declared.
This study was funded by Pacemed (US $200,000), Rio de Janeiro, Brazil.
The authors are grateful to all participants included in this study. The authors also thank Pacemed for stimulating the development of this study and for providing technical support.
- Scheffers IJ, Kroon AA, Schmidli J, Jordan J, Tordoir JJ, et al. (2010) Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J Am Coll Cardiol 56: 1254–1258.
- Bakris GL, Nadim MK, Haller H, Lovett EG, Schafer JE, et al. (2012) Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the Rheos Pivotal Trial. J Am Soc Hypertens 6: 152–158.
- Hoppe UC, Brandt MC, Wachter R, Beige J, Rump LC, et al. (2012) Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens 6: 270–276.
- Wallbach M, Halbach M, Reuter H, Passauer J, Lüders S, et al. (2016) Baroreflex activation therapy in patients with prior renal denervation. J Hypertens 34: 1630-1638. [crossref]
- Kiuchi MG, Chen S (2016) Renal sympathetic stimulation in patients with controlled hypertension and paroxysmal atrial fibrillation. Int J Cardiol 224: 394-397.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, et al. (2009) CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612.
2021 Copyright OAT. All rights reserv


