Studies employing percutaneous renal sympathetic denervation (RSD) [1-4] showed a reduction in both systolic and diastolic blood pressure (BP) among refractory hypertensive patients. As we described previously [5,6], the reduction in office BP was evident from the 1st month until one year after RSD in refractory hypertensive patients. A recent study in animals showed the pathology of radiofrequency-derived RSD during the time and provided important knowledge of the mechanisms resulting in sustained BP reduction, and reported that the nerve damage post radiofrequency ablation was greatest at 7 days, with maximum functional nerve damage sustained ≤30 days. Focal terminal nerve regeneration was observed only at the sites of ablation as early as 2 months and continued to 6 months [7]. Another study reported a substantial decrease in office systolic BP in relation to the number of ablation points at 6 months [8]. To date, there is no test to prove the acute efficacy of RSD.
As a hormone, epinephrine plays on closely all body tissues. Its actions vary by tissue kind and tissue manifestation of adrenergic receptors. Epinephrine acts by binding to a diversity of adrenergic receptors. Epinephrine is a nonselective agonist of altogether adrenergic receptors, comprising the major subtype’s α1, α2, β1, β2, and β3. Its activities are to increase peripheral resistance via α1 receptor-dependent vasoconstriction and to growth cardiac output via its binding to β1 receptors. Based on this information, we believe that epinephrine can be used to evaluate the acute response of BP and heart rate (HR) to RSD in uncontrolled hypertensive patients.
This transversal study involved 25 uncontrolled hypertensive subjects, was conducted in agreement with the Helsinki declaration and approved by the ethics committee of our institution. All subects sign up the informed consent term before inclusion. This study was piloted at the Hospital e Clínica São Gonçalo, Rio de Janeiro, Brazil. Patients were recruited from January 2015 to December 2016 from the Arrhythmias and Artificial Cardiac Pacing Service of the same hospital. Patients with the mixture of the subsequent criteria were successively enrolled: (i) mean 24-hour systolic ambulatory BP measurements (ABPM) of >130 mmHg and mean 24-hour diastolic ambulatory BP >80 mmHg, in use of at least 3 antihypertensive agents in the maximum doses prescribed or tolerated, being one of them a diuretic; (ii) a physically normal heart with an ejection fraction of >50% (Simpson’s method) to CRM, without ischemia, fibrosis area, or any other disease; (iii) age of 18 to 80 years, (iv) estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2 estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [9] (without microalbuminuria), and (vi) the capacity to read, understand, and sign the informed consent form and go to the clinical tests. The patients that presented any of the subsequent criteria were excluded: (i) pregnancy; (ii) valvular disease with significant adverse sequelae; (iii) unstable angina, myocardial infarction, transient ischemic attack or stroke previosly; (iv) renovascular abnormalities; (v) psychiatric disease; (vi) allergy to ionic contrast medium; (vii) the inability to be monitored clinically after the procedure; (viii) a known addiction to drugs or alcohol that disturbs the intellect; (ix) congestive heart failure presenting functional class II to IV symptoms according to New York Heart Association.
The general features of the the 25 uncontrolled hypertensive subjects at baseline are displayed in Table 1. The main goal of this study evaluated if RSD can reduce hypertensive response acutely even in the presence of a sympathomimetic agent. The RSD procedure has been defined in detail previously [10]. Before and after RSD the 2 mg of epinephrine intravenous were administered and we observed the variations in invasive BP and HR. The patients continued hospitalized in the ward for 24 h after the RSD.
Table 1. General features of patients at baseline.
General features of patients at baseline |
N |
25 |
Age, years |
53.0 ± 8.0 |
Body mass index, kg/m2 |
27.8 ± 3.0 |
Male sex (%) |
17 (68%) |
White ethnicity (%) |
15 (60%) |
Type 2 Diabetes Mellitus (%) |
6 (24%) |
Coronary artery disease |
5 (20%) |
Uncontrolled hypertension |
25 (100%) |
Creatinine, mg/dL |
0.90 ± 0.11 |
eGFR, mL/min/1.73 m² (CKD-EPI) |
99.0 ± 7.5 |
ACR, mg/g |
13.8 ± 5.5 |
Mean 24-hour ABPM, mmHg |
141.0 ± 6.0/90.4 ± 4.5 |
Antihypertensive agents |
|
ACEI/ARB |
25 (100%) |
Diuretics |
25 (100%) |
DHP Ca++ channel blockers |
25 (100%) |
β-blockers |
15 (60%) |
Spironolactone |
13 (52%) |
Clonidine |
12 (48%) |
Cardiac magnetic resonance |
|
Indexed LV mass/BSA, g/m2 |
128.3 ± 13.5 |
LVEF, % (Simpson) |
67.0 ± 6.8 |
LVEDD, mm |
45.0 ± 2.3 |
LVESD, mm |
36.2 ± 3.0 |
Indexed LA volume, mL/m2 |
27.0 ± 1.3 |
Values are presented as Mean ± SD or %; ABPM: ambulatory blood pressure measurements; ACEI: receptor inhibitor of angiotensin converting enzyme; ACR: albumin creatinine ratio; ARB: angiotensin receptor blocker; BSA: body surface area; DHP: dihydropyridyne; EF: ejection fraction; eGFR: estimated glomerular filtration rate; LA: left atrium; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; N: number of patients.
The results are expressed as a mean and standard deviation for normally distributed data and as median with interquartile range otherwise. All statistical tests were two-sided. Comparisons between two-paired values were performed with the paired t-test in cases of a Gaussian distribution and by the Wilcoxon test otherwise. Comparisons between more than two-paired values were made by repeated-measures analysis of variance or by Kruskal–Wallis analysis of variance as appropriate, complemented by a post-hoc test. A P-value <0.05 was considered significant. All statistical analyses were performed using the program Graphpad Prism v 7.0 (Graphpad Software, La Jolla, CA, USA).
At baseline, the mean invasive systolic/diastolic BP were 128.0 ± 6.9/87.1 ± 4.1 mmHg and the mean HR was 71.0 ± 8.3 bpm. After 2 mg of ephedrine intravenous and before RSD, the mean invasive systolic/diastolic BP were 189.4 ± 10.9/134.5 ± 6.9 mmHg and the mean HR was 131.4 ± 9.6 bpm. Acutely post RSD procedure the mean invasive systolic/diastolic BP were 110.3 ± 8.5/73.3 ± 2.4 mmHg and the mean HR was 61.3 ± 7.7 bpm. The same infusion of 2 mg of ephedrine intravenous after RSD, the mean invasive systolic/diastolic BP were 144.3 ± 6.2/93.6 ± 5.2 mmHg and the mean HR was 105.2 ± 6.7 bpm. All the comparisons between the same parameter were significant (P<0.0001), as shown in Figure 1.
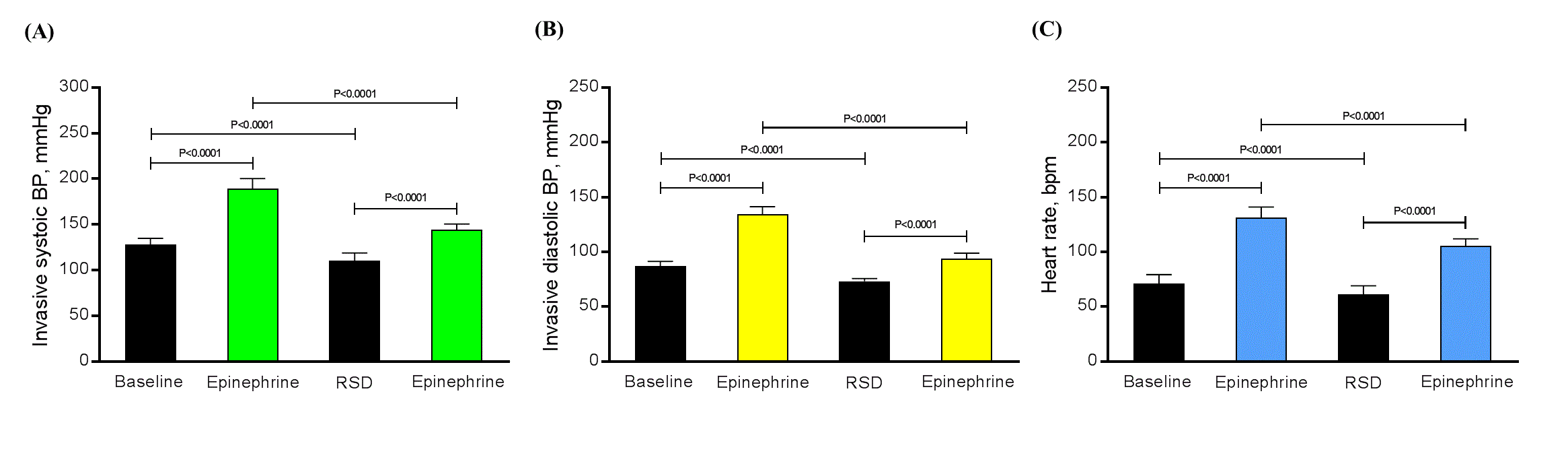
Figure 1. The variation in mean invasive systolic/diastolic BP (A) and (B), respectively, as well as, in the mean heart rate (C) at baseline, after 2 mg of ephedrine intravenous and before RSD, immediately after RSD, and post 2 mg of ephedrine intravenous post RSD. N = 25 uncontrolled hypertensive patients.
In conclusion, our study shows that the RSD reduces the hyperactive response in mean invasive systolic/diastolic BP and the rise in the acutely HR even in the presence of epinephrine intravenous. These findings suggest that this fast and acute test in the future may be used, but other studies with a large number of patients should be performed.
None declared.
This study was funded by Pacemed (US $100,000), Rio de Janeiro, Brazil.
The authors are grateful to all participants included in this study. The authors also thank Pacemed for stimulating the development of this study and for providing technical support.
- Krum H, Schlaich M, Whitbourn R (2009) Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275-1281. [Crossref]
- Symplicity HTN-1 Investigators (2011) Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension 57: 911-917. [Crossref]
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, et al. (2010) Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903-1909. [Crossref]
- Voskuil M, Verloop WL, Blankestijn PJ, Agostoni P, Stella PR, et al. (2011) Percutaneous renal denervation for the treatment of resistant essential hypertension; the first Dutch experience. Neth Heart J 19 319-323. [Crossref]
- Kiuchi MG, Maia GL, de Queiroz Carreira MA, Kiuchi T, Chen S, et al. (2013) Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J 34: 2114-2121. [Crossref]
- Kiuchi MG, Chen S, Andrea BR, Kiuchi T, Carreira MA, et al. (2014) Renal sympathetic denervation in patients with hypertension and chronic kidney disease: does improvement in renal function follow blood pressure control? J Clin Hypertens (Greenwich) 16: 794-800. [Crossref]
2021 Copyright OAT. All rights reserv
- Sakakura K, Tunev S, Yahagi K, O'Brien AJ, Ladich E, et al. (2015) Comparison of histopathologic analysis following renal sympathetic denervation over multiple time points. Circ Cardiovasc Interv 8: 2. [Crossref]
- Ghadri JR, Gaehwiler R, Jaguszewski M, Sudano I, Osipova J, et al. (2015) Impact of local vascular lesions assessed with optical coherence tomography and ablation points on blood pressure reduction after renal denervation. Swiss Med Wkly 145. [Crossref]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604-612.
- Kiuchi MG, E Silva GR, Paz LM, Chen S, Souto GL (2016) Proof of concept study: renal sympathetic denervation for treatment of polymorphic premature ventricular complexes. J Interv Card Electrophysiol 47: 221-229. [Crossref]
Editorial Information
Editor-in-Chief
Massimo Fioranelli
Guglielmo Marconi University
Article Type
Short Communication
Publication history
Received date: January 01, 2017
Accepted date: January 17, 2017
Published date: January 20, 2017
Copyright
© 2017 Márcio Galindo Kiuch. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Kiuchi MG and Chen S (2016) Acute epinephrine test after renal sympathetic denervation in uncontrolled hypertensive patients. J Integr Cardiol 3: DOI: 10.15761/JIC.1000208.
Corresponding author
Márcio Galindo Kiuchi
Division of Cardiac Surgery and Artificial Cardiac Stimulation, Department of Medicine, Hospital e Clínica São Gonçalo, Rua Cel. Moreira César, 138 - Centro, São Gonçalo, Rio de Janeiro 24440-400, Brazil, Tel/Fax: +55 (21) 26047744

Figure 1. The variation in mean invasive systolic/diastolic BP (A) and (B), respectively, as well as, in the mean heart rate (C) at baseline, after 2 mg of ephedrine intravenous and before RSD, immediately after RSD, and post 2 mg of ephedrine intravenous post RSD. N = 25 uncontrolled hypertensive patients.
Table 1. General features of patients at baseline.
General features of patients at baseline |
N |
25 |
Age, years |
53.0 ± 8.0 |
Body mass index, kg/m2 |
27.8 ± 3.0 |
Male sex (%) |
17 (68%) |
White ethnicity (%) |
15 (60%) |
Type 2 Diabetes Mellitus (%) |
6 (24%) |
Coronary artery disease |
5 (20%) |
Uncontrolled hypertension |
25 (100%) |
Creatinine, mg/dL |
0.90 ± 0.11 |
eGFR, mL/min/1.73 m² (CKD-EPI) |
99.0 ± 7.5 |
ACR, mg/g |
13.8 ± 5.5 |
Mean 24-hour ABPM, mmHg |
141.0 ± 6.0/90.4 ± 4.5 |
Antihypertensive agents |
|
ACEI/ARB |
25 (100%) |
Diuretics |
25 (100%) |
DHP Ca++ channel blockers |
25 (100%) |
β-blockers |
15 (60%) |
Spironolactone |
13 (52%) |
Clonidine |
12 (48%) |
Cardiac magnetic resonance |
|
Indexed LV mass/BSA, g/m2 |
128.3 ± 13.5 |
LVEF, % (Simpson) |
67.0 ± 6.8 |
LVEDD, mm |
45.0 ± 2.3 |
LVESD, mm |
36.2 ± 3.0 |
Indexed LA volume, mL/m2 |
27.0 ± 1.3 |
Values are presented as Mean ± SD or %; ABPM: ambulatory blood pressure measurements; ACEI: receptor inhibitor of angiotensin converting enzyme; ACR: albumin creatinine ratio; ARB: angiotensin receptor blocker; BSA: body surface area; DHP: dihydropyridyne; EF: ejection fraction; eGFR: estimated glomerular filtration rate; LA: left atrium; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; N: number of patients.

