A number of studies in laboratory rodents have shown how glucocorticoid hormones (GC) alter thymus functionality. Conversely, how GC influence normal human thymus functionality is largely unknown for obvious ethical reasons. Current guidelines state that healthy pregnant women destined to preterm delivery should be considered for antenatal GC (AGC) administration before delivery to facilitate fetal lung maturation. We reasoned that this procedure exposes fetal thymus to GC and investigated AGC impact on fetal thymus functionality in 15 pregnant women (8 on GC and 7 untreated) by measuring changes occurring in major T cell subset and recent thymic emigrant frequency in newborn’s cord blood. Fetal thymus morphology was assessed sonographycally. Despite AGC reduced thymic transverse diameter and perimeter (p< 0.05 both parameters), no measurable change occurred in recent thymic emigrant frequency and major T cell subset, including regulatory T cell (CD25+Foxp3+CD4+T cells, T-regs). Conversely, AGC reduced the proportion of CD45RA+ T-regs (78.4 ± 7.15% and 67.8 ± 5.45%, mean ± SD, untreated and treated subjects, respectively, p<0.02) and this effect was selective for T-regs, as CD45RA expression in conventional CD4+ T cells was not modified by AGC (92.9 ± 3.5 % and 94.4 ± 2.4 %, mean ± SD, untreated and treated subjects, respectively. However, CD45RA modulation did not affect Treg functionality. The accumulation of T-regs with a CD45RA− phenotype induced by AGC may reflect a reduced T-reg output from thymus followed by homeostatic expansion in the periphery for T-reg pool replenishment, as in adults, or a thymic export of not yet mature T-regs that maintain their CD45RA− intrathymic phenotype.
antenatal glucocorticoids, regulatory T cells, cord blood, human thymus
List of abbreviations
AGC: Antenatal GC; CS: Cesarean Section; CB: Cord Blood; CBMCs: Cord Blood Mononuclear Cells; FITC: Fluorescein Isothiocyanate; GA: Gestational Age; GC: Glucocorticoid Hormones; IL-2: Interleukin-2; MoAbs: Monoclonal Antibodies; ECD: PE-texas red; PC5: PE/Cy5; PE: Phycoerythrin; RTE: Recent Thymic Emigrants; T-regs: Regulatory T Cells; US: Ultrasound
While endogenous glucocorticoid hormones (GC) of the adrenal cortex play an important role in thymocyte selection and are pivotal for development of a fully responsive T cell repertoire [1, 2] several studies have reported on detrimental effects of exogenous GC administration on normal thymus functionality since the early ‘70s in laboratory rodents [3-5]. These studies demonstrated that GC administration produces a profound rearrangement of thymocyte subset distribution that is consistent with a selective depletion of immature cortical thymocytes and an enrichment of more mature thymocyte subsets in the medulla. Since these in vivo data derive from studies in laboratory rodents, being healthy human subjects obviously not amenable to GC treatment and thymus removal, there is minimal information about how normal human thymus responds to GC administration. In fact, the only setting in which human thymus is available for analysis after GC administration is myasthenia gravis, an autoimmune disorder in which thymic ablation following GC administration is a common therapy for many patients since the thymus is believed to be the initiation organ of pathogenesis [6]. We and others have shown that GC administration in myasthenia gravis patients causes a profound rearrangement of thymocyte subset distribution that is consistent with a selective depletion of immature cortical thymocytes and a relative enrichment of more mature thymocyte subsets in the medulla [7-9]. These data are consistent with studies showing that human immature cortical thymocytes do undergo apoptosis after treatment with GC in vitro [9-11]
The insights obtained in the myasthenia gravis setting, however, are confounded by the autoimmune features of the disease and, perhaps most importantly, by the fact that myasthenia gravis patients are adults and thymus involutes since the age of ~1 year and is gradually replaced by adipose tissue [12,13] .
Antenatal GC (AGC) are currently administered few days before delivery to otherwise healthy pregnant women at risk of preterm delivery (i.e., between 34 and 37 weeks) to minimize neonatal morbidity and mortality, and favour lung maturation [14]. Animal studies have shown that AGC administration induces evident macroscopic and microscopic structural changes on fetal thymus [15,16] indicating that AGC administration is detrimental for fetal thymus development. Consistently, chest x ray pictures taken early after delivery in human newborns have shown a significantly smaller fetal thymus in newborns whose mothers received AGC [17]. Thus, we reasoned that AGC administration for pregnancies at risk for early preterm delivery represents a suitable setting for exploring the effects of GC on normal young human thymus. Direct analysis of the thymus in healthy newborns at birth is obviously ethically impossible. However, since fetal immune system is exposed to a very limited antigenic load during normal pregnancy, the contribution of peripherally expanded T cells to the composition of T cell pool in cord blood (CB) should be negligible. This makes it realistic to assume that the composition of T cell pool in CB reflects fetal thymus functionality. There are few data on the effects of AGC administration on CB immune cells. In one report, AGC administration upmodulated T cell frequency [18], whereas in another report it reduced the amount of total lymphocytes and of certain T cell subsets [19].
In the present study, we measured the effect of AGC administration on the distribution of the major T cell populations in CB, including the so-called recent thymic emigrants (RTE) the best surrogate indicator of thymic functionality. Next, we focused on changes occurring in regulatory T cells (T-regs) since the thymus is a primary source of this T cell subset [20-22]. Studies in humans have clearly defined that T-regs mature in the thymus as CD4+CD25+CD45RA+ T cells and are then released into the periphery where they adopt a memory CD45RO+ CD45RA− phenotype profile upon antigen contact [23,24]. Consistently, memory-like T-regs, which represent the majority of T-regs in adult blood, are quite scarce in CB [25,26] making CB T-regs a suitable model to explore the activity of GC on their intrathymic educational development.
As alluded to above, chest x ray pictures showed a significantly smaller fetal thymus in newborns of mothers on AGC [17]. With advances in ultrasound (US) technology, a non-invasive tool that is currently used for prenatal diagnosis of a varieties of fetal abnormalities, the assessment of prenatal thymus morphological changes has become much more feasible. For example, US has been successfully used to assess thymic involution as integral part of the fetal inflammatory response to a hostile environment in the context of intrauterine infection in women with preterm labour [27-29]
Interestingly, this clinical setting is characterized by an exaggerated production of endogenous GC, suggesting that US is able to detect GC-induced thymus morphological changes. Thus, in the present study we used US to document morphological changes occurring in fetal thymus after AGC administration and to relate them to changes occurring in CB immune cell distribution.
Patients
A prospective observational study was conducted in 15 (8 on GC and 7 untreated) pregnant women admitted between October 2014 and November 2015 to the Department of Obstetrics and Gynecology. The study population was selected from pregnant women attending for programmed cesarean section (CS) and who satisfied the following criteria: a well-defined gestational age (GA) confirmed or modified by a first trimester measure of crown-rump length by US, singleton and uncomplicated pregnancy. Exclusion criteria were: intrauterine growth restriction, congenital and chromosomal abnormalities, twin pregnancy, history of fetal complications and maternal disease affecting fetal growth and immune system. Table 1 shows the demographic data from the included subjects. Maternal characteristics, indication for CS, GA at delivery, Apgar score, birthweight, neonatal days of hospitalization and presence (lack of) of fetal infection were well balanced across the two groups. AGC treatment consisted of 24 mg of betamethasone given as two intramuscular injections administered at 24 hour intervals within 7 days before delivery [14]. US examination was performed using a GE Voluson E8 (GE Medical Systems, Zipf, Austria) US machine. Patients underwent the sonographic evaluation at least 72 hours after AGC administration and within 24 hours before the programmed CS to assess fetal biometry (biparietal diameter, abdominal circumference and femur length. Estimated fetal weight was obtained using Hadlock et al., formula [30]; the thymic biometry were assessed in the three-vessel view: perimeter [31] and maximum transverse diameter of the thymus were measured by placing a line cursor perpendicular to the line connecting the sternum and the spine [32].
Ethics Committee approval and informed consent were obtained from all women.
CB mononuclear cell isolation
After the delivery, the cord was clamped and ~12 ml of CB were collected into heparinized tubes. CB mononuclear cells (CBMCs) were purified via standard density gradient centrifugation using Ficoll–Hypaque (Sigma-Aldrich, Munich, Germany), as described [33]. CBMCs were washed, suspended in Ca+Mg+ free phosphate buffer saline (PBS) and processed immediately for flow cytometry analysis.
Flow cytometry analysis
Flow cytometry analysis of cell surface phenotype and the intracellular expression of the transcription factor Foxp3 was performed using appropriate combinations of fluorochrome-conjugated monoclonal antibodies (MoAbs). Fluorescein isothiocyanate (FITC)- CD45RA, CD56, and TCR γd MoAbs were from Beckman Coulter (Miami, FL). FITC-Foxp3 MoAb (clone PCH101) was from eBioscience, (San Diego, CA). Phycoerythrin (PE)- CD3, CD31, CD45RO, and CD69 Moabs were from Beckman Coulter. PE-CD25 MoAb was from BD Biosciences (Mountain View, CA). PE-texas red (ECD)- CD3, CD4, CD62L, and CD45RA MoAbs were from Beckman Coulter. PE/Cy5 (PC5)- CD4, CD8, and CD19 MoAbs were from Beckman Coulter. CBMCs were incubated with pre-titrated MoAbs for 20 min at room temperature, washed in cold PBS and finally suspended for flow cytometry analysis. Because Foxp3 is an intracellular antigen, cells have to be permeabilized to allow MoAb entry. With permeabilized lymphocytes, MoAbs can give increased background fluorescence, possibly due to entry of free fluorochrome and/or MoAb reactivity with charged or polar internal molecules, that cannot be correctly evaluated by the conventional isotype staining.
Thus, for intracellular Foxp3 staining, CBMCs were first stained with MoAbs to surface markers CD4, CD25 and CD45RA. CBMCs were then fixed and permeabilized using Foxp3 staining buffer (eBioscience), according to manufacturer’s instructions. To demonstrate specificity of staining an isoclonic control was used. Briefly, the binding of FITC-Foxp3 MoAb was blocked by preincubation of the fixed/permeabilized cells with unlabelled Foxp3 MoAb antibody (10 fold molar excess) prior to staining with the FITC-Foxp3 MoAb [33]. Interleukin-2 (IL-2) production by T-regs was detected using PE-anti-IL-2 MoAb (BD Biosciences), as described [34]. Briefly, after surface staining with CD4 CBMCs were washed and then stimulated with 10 nM phorbol 12-myristate acetate (Sigma-Aldrich, Munich, Germany) and 1µM ionomycin (Sigma) in the presence of brefeldin (10 µg/ml), Sigma) in RPMI medium supplemented with 5% fetal bovine serum (FBS) at 37°C for 4 hours. After washing with cold PBS, CBMCs were fixed and permeabilized with Foxp3 staining buffer and finally stained with Foxp3 and IL-2 MoAbs. In some experiments, CBMCs were also stained with CD45RA MoAb before fixation/permeabilization.
After staining, the cells were analysed in an EPICS-XL (Beckman Coulter) flow cytometer. Cells with forward and side scatter properties of monocytes and lymphocytes were gated. For each cell population, the cut-off level was set to include 2% of the isotypic control cells, except for Foxp3 whose background was assessed using the isoclonic control as described above. The percentage of positive cells above the cutoff level was recorded. List mode data were analysed using EXPO 32TM software. A minimum of 5000 cells of interest was acquired for each sample. A forward scatter/side scatter based gate was used to identify lymphocytes and monocytes among CBMCs and calculate the percentage of each cell population of interest. Flow cytometry analyses were conducted by two independent observers to limit the impact of the investigator’s interpretation on flow data.
Statistical analysis
Two sample or paired t-test was used to determine the significance of differences in the distribution of the various immune cell subsets. All tests were two-sided. Statistical analyses were performed on non-transformed data using the Statistica software, version 7 (StatSoft).
Effects of AGC administration on thymus size
The mean transverse diameter and the mean perimeter of the study group (40.7 ± 4.9 mm and 126.9 ± 8.7, respectively) were in the normal range according to Cho et al. [32] and Gamez et al. [35] (Table 2). Changes induced by AGC administration in fetal thymus size are summarized in Table 2. AGC administration significantly reduced thymus size as assessed by measuring the transverse diameter and perimeter. Transverse diameter and perimeter of the fetal thymus increases according to fetal GA in a linear manner [31,32,35]. Thus, since the GA of untreated and AGC treated subjects was different (Table 1), GA might act as a confounding factor on measurements. To take into account a possible influence of this variable on thymic size, we adjusted for GA by calculating the ratio between the thymus transverse diameter and GA (in days), and the thymus perimeter and GA (in days). As shown in Table 2, the detrimental effect of AGC administration on thymus size remained evident after correction for GA. This allowed to conclude that AGC administration was effective in reducing thymus size.
Table 1. Characteristics of the study population.
|
Untreated
(n=7) |
AGC treated
(n=8) |
P value |
Maternal age |
30.1 ± 3.29 |
34.9 ± 5.87 |
N.S. |
Parity |
1 ± 0 |
1 ± 0 |
N.S. |
BMI |
24.10 ± 4.28 |
23.31 ± 2.29 |
N.S. |
CS indication |
|
|
|
Fetal malposition |
1 (14.3) |
0 |
- |
Previous CS |
5 (71.4) |
6 (75) |
N.S. |
Maternal indication for CS |
1 (14.3) |
2 (25) |
N.S. |
GA at delivery |
274 ± 2.3 |
264 ± 5.3 |
<0.05 |
Apgar < 7 |
0 (0) |
0 (0) |
- |
Birthweight (g) |
3,203.9 ± 335.3 |
2,877.5 ± 319.9
|
N.S. |
NICU admission |
0 (0) |
0 (0) |
- |
Neonatal infection |
0 (0) |
3 (37.5) |
- |
Values are reported as mean ± SD or n (%). BMI, body mass index; CS, Cesarean section; GA, gestational age; NICU, neonatal intensive care unit; N.S., not significant
Table 2. Values of fetal thymus in untreated and AGC treated subjects.
|
Untreated
(n=7) |
AGC treated
(n=8) |
P value |
Thymus diameter (mm) |
40.7 ± 4.9 |
33.6 ± 2.84 |
< 0.05 |
Thymus perimeter (mm) |
127.0 ± 8.7 |
102. 5 ± 2.84 |
< 0.05 |
Thymus diameter/days of GA |
0.15 ± 0.018 |
0.13 ± 0.009 |
< 0.05 |
Thymus perimeter/days of GA |
0.46 ± 0.033 |
0.39 ± 0.012 |
< 0.05 |
Values are reported as mean ± SD; GA, gestational age.
Effects of AGC administration on immune cell populations
The percentages of cells positive for the specific markers were calculated, with total lymphocytes–which were identified on the basis of a forward scatter/side scatter based gate– as the denominator (Table 3). Thus, data are presented as percentage of gated lymphocytes expressing the markers that together define the subset of interest. Following AGC administration, no statistically significant changes were observed in the percentage of total T cells and major T cell subsets, although in AGC treated subjects there was a measurable decrease in CD4+/CD8+ T cell ratio due to a slightly reduced CD4+ T cell frequency and a correspondent slight increase in CD8+ T cell frequency (Table 3). We also explored the effect of AGC administration on CD4¯CD8¯ T cells a T cell subset that has been proposed to originate in the thymus by escaping negative selection and that may contribute to pediatric autoimmunity [36], and gd T cells, a T cell subset that plays a major role in local immunosurveillance [37]). AGC administration did not impact on either T cell population (Table 3).
Table 3. Distribution frequency of the immune cell populations in the CB of untreated and AGC treated subjects.
Cell population |
Untreated
(n=7) |
AGC treated
(n=8) |
T cells |
75.4 ± 3.3 |
75.1 ± 6.6
|
CD4+T cells |
56.2 ± 6.1 |
52.1 ± 7.1
|
CD8+T cells |
14.7 ± 2.6 |
17.4 ± 2.3 |
CD4+/CD8+T cell ratio |
4.0 ± 1.7 |
2.9 ± 0.9 |
CD4–CD8– T cells |
2.0 ± 0.7 |
1.5 ± 0.5
|
gd T cells |
1.6 ± 0.6 |
1.1 ± 0.3 |
CD45RA+CD4+ T cells |
47.9 ± 6.0 |
43.3 ± 6.3 |
CD45RA+CD8+ T cells |
13.1 ± 2.6 |
15.5 ± 2.5 |
RTE |
32.0 ± 7.4 |
34.9 ± 5.8 |
B cells |
9.1 ± 1.9 |
11.3 ± 4.2 |
NK cells |
6.7 ± 3.3 |
5.9 ± 3.0 |
Values are reported as mean ± SD.
RTE, recent thymic emigrants (CD4+CD45RA+CD62L+CD31+ T cells).
We reasoned that evaluation of the distribution of these major T cell subsets might not be suited to measure changes occurring in thymus output following AGC administration and subtler changes in thymus functionality might have been missed. Thus, we focused on the T cell subsets that exhibit a naïve-like phenotype and are currently regarded as the most direct indicators of thymic functionality, i. e., CD45RA+CD4+ and CD45RA+CD8+ T cells [38], and on the so-called recent thymic emigrants (RTE) identified as CD45RA+CD62L+CD31+CD4+ T cells [39, 40]. Neither naïve-type CD4+ and CD8+ T cell nor RTE frequency was modified by AGC administration (Table 3). Also frequencies of other major immune cell populations namely, B cells and NK cells, that were assessed to complete the picture of the whole CB lymphoid cell population, were not impacted by AGC administration.
Effects of AGC administration on T-regs
We initially ascertained that the T-regs that were phenotypically identified as CD4+CD25+Foxp3+ T cells (Figure 1a) were bona fide T-regs since even the presence of Foxp3, the master regulator of T-reg development and function, does not necessarily associate with suppressive activity: Foxp3 can be induced by TCR stimulation in conventional Foxp3−CD4+ T cells without conferring suppressive activity [41,42]. Thus, it was mandatory to test the regulatory ability of phenotypically identified T-regs in a functional assay. Unfortunately, testing T-reg suppressive activity in a classical T cell proliferation inhibition assay was not feasible, being restricted by cell numbers. It is known that T-regs are poor producers of IL-2 due to the ability of Foxp3 to hamper transcription from cytokine promoter whereas Foxp3+ activated conventional CD4+ T cells, i.e., non-T-regs, maintain their ability to produce IL-2 [42,43]. Thus, to validate T-reg identity we evaluated a distinct feature of T-regs i.e., their inherent inability to produce IL-2 [33, 34]. A representative experiment carried out in one out of four untreated subjects that were used for the assay is depicted in Figure 1b. Most putative T-regs did not produce IL-2. Thus, we concluded that the cells we phenotypically defined as T-regs by the expression of CD4, CD25 and Foxp3 could be convincingly identified as T-regs. Comparable results were obtained testing four AGC treated subjects (not shown).
Having established the T-reg nature of the CD4+CD25+Foxp3+ T cells in CB of untreated and AGC treated subjects, we calculated the percentages of cells positive for these specific markers within CD4+ T cells –which were identified on the basis of the forward scatter/side scatter based gate and CD4 expression– as the denominator in the two groups. As shown in Figure 1c, AGC administration did not significantly modify T-reg frequency.
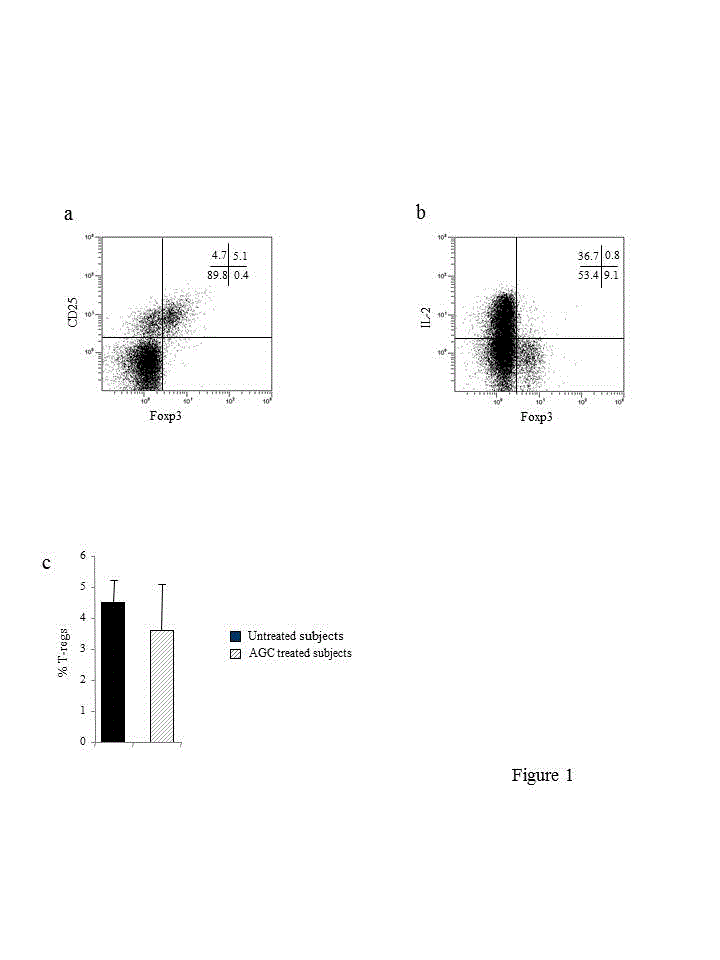
figures 1. . a, dual colour dot plot exemplifying flow cytometry strategy used to identify T-regs by concomitant expression of CD25 and Foxp3. The plot is gated on CD4+T cells. Quadrant markers are set based on the autofluorescence control and verified with unlabelled blocking anti-Foxp3 antibody (isoclonic control) as described in Materials and Methods. Numbers represent the percent of cells residing in each quadrant. b, compilation of T-reg data from untreated and AGC treated subjects. c, dual colour dot plot showing flow cytometry strategy used to identify IL-2 production upon polyclonal stimulation by putative T-regs. The plot is gated on CD4+T cells. Numbers represent the percent of cells residing in each quadrant. The proportions of IL-2 producing cells within Foxp3+ cells was calculated using the following formula:
% of cells in upper right quadrant / (% of cells in upper right quadrant) + (% of cells in lower right quadrant) × 100. In the subject shown here a marginal proportion (8.1%) of Foxp3+ cells produced IL-2, indicating that most Foxp3+ cells were indeed T-regs.
CD45RA has originally been indicated as a marker of T-regs of direct thymus origin [44] and studies have indicated that most CB T-regs express CD45RA, in line with their recent thymus derivation and naïve, non-antigen experienced, nature [25]. CD45RA expressing T-regs were enumerated by flow cytometry (Figure 2a), and found to represent the largest T-reg subset both in untreated and AGC treated subjects. However, the proportion of CD45RA expressing T-regs was significantly reduced by AGC administration (Figure 2a,b). We next subfractionated conventional CD4+T cells on the basis of CD45RA expression to explore whether the effect of AGC administration on CD45RA+ T-regs was selective. Both in untreated and AGC treated subjects most conventional CD4+T cells expressed CD45RA, and the proportions were not affected by AGC administration (92.9 ± 3.5 % and 94.4 ± 2.4 %, mean ± SD, untreated and AGC treated subjects, respectively). To validate T-reg identity of the CD45RA+ and CD45RA− T-reg subsets we evaluated their ability to produce IL-2, as outlined above. As summarized in Table 4, both CD45RA+ and CD45RA− T-regs showed equivalent frequencies of IL-2-producing cells in untreated and AGC treated subjects, thereby demonstrating that CD45RA+ and CD45RA− T-regs were both mostly composed of suppressive populations, irrespective of AGC administration.
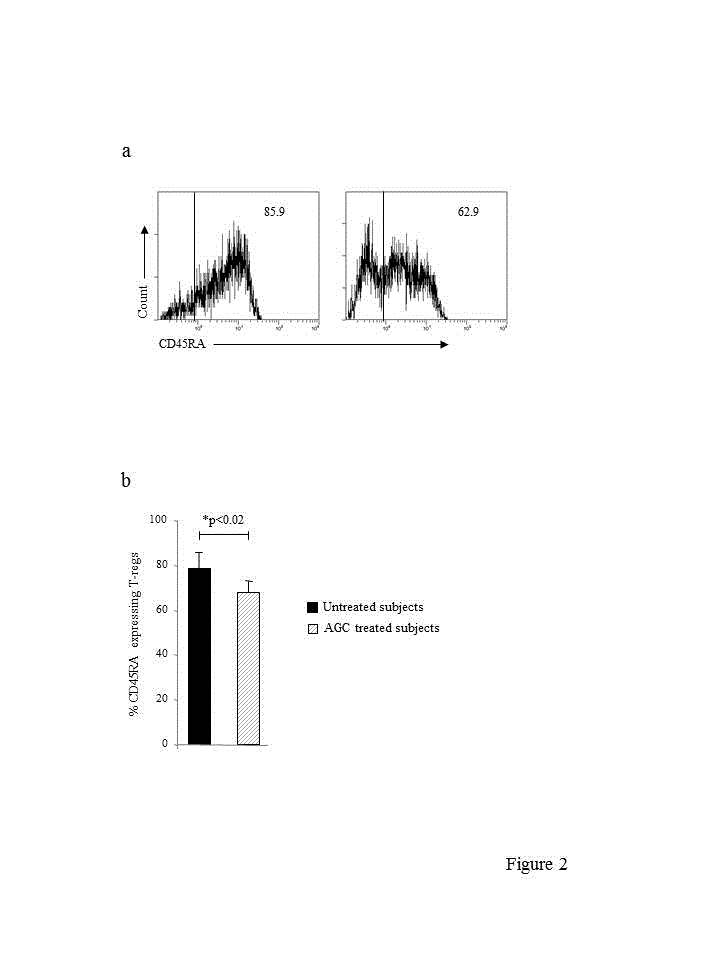
figures 2. . a, representative single colour histogram plots showing CD45RA expressing T-regs in an untreated (left panel) and AGC treated (right panel) subject. The plots are gated on CD4+CD25+Foxp3+ T cells. Markers are set based on the autofluorescence control. Numbers represent the percent of cells expressing CD45RA. b, compilation of CD45RA expressing T-reg data from untreated and AGC treated
Table 4. IL-2-producing cells in CD45RA+ and CD45RA¯ T-regs in untreated and AGC treated subjects.
|
CD45RA+
(%) |
CD45RA-
(%) |
Untreated |
8.25 ± 6.15 |
12.70 ± |
AGC treated |
8.05 ± 2.76 |
12.95 ± 1.48 |
Studies indicate that in adults CD45RA+ T-regs differ from their CD45RA− counterpart in terms of CD25 and Foxp3 expression level [24,44]. Thus, we compared CD25 and Foxp3 expression level in CD45RA+ and CD45RA− T-regs in untreated and AGC treated subjects. We found that CD45RA+ T-regs were characterized by a significantly lower expression level of CD25 and Foxp3 as compared to their CD45RA− counterpart irrespective of AGC administration (Table 5), as in adults.
Table 5. CD25 and Foxp3 expression intensity in CD45RA+ and CD45RA ̶ T-regs in untreated and AGC treated subjects.
|
|
CD25 (MFI) |
|
|
Foxp3 (MFI) |
|
|
CD45RA+ |
p* |
CD45RA̶ |
CD45RA+ |
p |
CD45RA̶ |
Untreated |
6.64 ± 2.66 |
0.02 |
7.50 ±3 |
5.80 ± 0.85 |
0.03 |
6.96 ± 1.58 |
AGC treated |
8.67 ± 3.0 |
0.0028 |
10.13± 3.33 |
4.82 ± 1.79 |
0.06 |
6.55 ± 3.39 |
MFI, mean fluorescence intensity; mean fluorescence channel was used as a measure of the fluorescence intensity as distribution of fluorescent populations were unimodally distributed.*Student’s t test for paired samples.
Despite a number of studies in animal models [3-5] and in patients undergoing therapeutic thymectomy for MG and/or thymoma [7-9] had shown that exogenous GC administration affects thymus functionality, obvious ethical reasons have prevented similar investigations to be performed in healthy young human subjects. Over the past four decades, AGC administration to healthy pregnant women destined to preterm delivery has demonstrated efficacy in reducing neonatal mortality and morbidity by facilitating fetal lung maturation [14]. This clinical setting offers the unique opportunity to investigate the effect of exogenous GC on the functionality of healthy young human thymus using the assessment of CB T cells as a correlate of thymic activity.
Here we found that AGC administration impacted on fetal thymus that appeared reduced in size by US measurement, in accord with earlier data [17]. The extent of reduction made the diameter of the thymus of AGC treated subjects lower than that reported by Cho et al. [32] for fetuses of analogous GA. It is likely that this effect reflects the rapid destruction of a large amount of thymus cells ensuing AGC administration originally demonstrated in laboratory animals [15,16]. This finding is also in line with in vitro studies showing that human thymocytes are highly sensitive to the cytolytic effect of GC [10,11].
Despite the evident changes in thymus size, there was no impact on thymic output, as evidenced by the absence of significant changes in the whole T cell pool and its subsets, including those that are most indicative of thymic export namely, the naïve T cell pool and the CD31+ cells among naïve T cell pool i.e., RTE. It is possible that the lack of an evident impact of AGC administration on thymic functionality in the present setting may merely be due to either a too low AGC total dosage, short administration time, or both. Additionally, possible alterations of the thymic functionality after AGC administration may have passed unnoticed because either they were too small to be reflected as measurable changes in the CB T cell pool, may have reverted by the time of CB examination because of the known rapid decline of plasma GC concentration [45], or both.
AGC total dosage and administration modality are inherent limitations of the present study since a different treatment schedule would be practically and ethically difficult to perform. In contrast with our findings, one study reported a significant increase in T cell frequency after AGC administration [18]. We can only conjecture as to why our results differ. A ccomparison is difficult because of factors like the immunofluorescence methods used in the present study (a mononuclear cell purification step before immunophenotyping) and the earlier study (a lysed whole blood procedure). Differences in the clinical setting may also contribute to the discrepancy since in that paper the effects of AGC administration were tested in peripheral blood of preterm newborns in the presence of various maternal comorbidities and not in CB of newborns of healthy women, as in the present study. Notably, Chabra et al. [19] reported a significant reduction in the absolute number of CD4+ T cells after AGC administration. We did not calculate absolute numbers of CB cells. However, this result may be in line with the modest reduction in the proportion of CD4+ T cells we observed here.
Despite AGC administration had an overall scarce impact on general thymic functionality, focusing on T-regs showed that, conversely, AGC administration did modulate T-reg homeostasis by reducing the frequency of the CD45RA+ T-reg subset. CD45RA expressing T-regs are the prominent T-reg population in CB where they represent unprimed naive T-regs of direct thymic derivation [25]. Consistently, studies in adults show that (the few) CD45RA expressing T-regs are unprimed naive cells of direct thymic origin whereas their (more abundant) CD45RA− counterpart represents T-reg cells mostly generated in the periphery [44]. In adults, these CD45RA− T-regs compensate for the physiological reduction of T-reg production by the aging thymus and maintain the homeostasis of the T-reg pool [44]. Because the frequency of the whole T-reg population i.e., the sum of CD45RA+ and CD45RA ̶ cells, was not modified by AGC administration, one might infer that AGC administration reduces T-reg output from thymus and that a homeostatic expansion in the periphery fills the T-reg pool, as in adults. The lower CD25 and Foxp3 expression level in CD45RA+ T-regs as compared to their CD45RA− counterpart, typically seen in adult T-regs [44], supports this view.
However, we cannot exclude the possibility that the relative abundance of CD45RA− T-regs following AGC administration might be a consequence of an altered intrathymic development of T-regs. Several studies [3-5] have demonstrated that GC administration targets immature thymocytes producing an enrichment of more mature thymocyte subsets in the medulla as a consequence of a compensatory accelerated transition of the earlier stages of development to replenish the depleted compartment. Thymocytes, including T-regs, have to undergo a finely regulated sequence of developmental steps in the cortex and medulla to finish the conversion from CD45RA− immature to CD45RA+ mature T-cells just before leaving the thymus [25,46,47] Thus, it may be speculated that the disorganized thymic environment ensuing AGC administration favours an enhanced egress of immature intrathymic CD45RA− T-regs into the periphery. In this scenario, since the proportion of conventional CD45RA+CD4+ T cells in CB was not affected by AGC administration, it may be speculated that GC have a predilection for intrathymic T-reg development.
AGC administration has been related to childhood asthma [48]. Because T-regs contribute to the control of allergen-specific immune responses in several major ways [49], one may suspect that AGC favors the development of asthma in childhood as a consequence of changes in T-reg subsets. This seems not to be the case, since both CD45RA− and CD45RA+ T-regs subsets were equally functional in the CB of the newborns of both untreated women and women on AGC, implying that the overall regulatory capability of T-regs in CB is not affected by AGC administration.
In conclusion, we report for the first time that AGC administration for fetal lung maturation in healthy women before delivery does not measurably affect global fetal thymus output, yet it induces sizeable changes in the relative proportion of CD45RA− and CD45RA+ T-regs. Whether the shift in the distribution of these two T-reg subsets has any biological and clinical effect remains unknown. Follow-up into childhood is needed to inform later outcomes of treatment.
V. C. and M. D. V performed research and analyzed data; I. M., S. B, and P. C. contributed clinical data and performed statistical analysis; P. R., A. B., and A. F. designed research and wrote the paper.
The authors declare that they have no conflict of interest.
- Ashwell JD, Lu FW, Vacchio MS (2000) Glucocorticoids in T cell development and function. Annu Rev Immunol 18: 309-345. [Crossref]
- Mittelstadt PR, Monteiro JP, Ashwell JD (2012) Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest 122: 2384-2394. [Crossref]
2021 Copyright OAT. All rights reserv
- Claman HN (1972) Corticosteroids and lymphoid cells. N Engl J Med 287: 388-397. [Crossref]
- Bach JF, Strom TB. The mode of action of immunosuppressive agents. 2nd ed. Amsterdam: Else-vier; 1985.
- Cohen JJ (1992) Glucocorticoid-induced apoptosis in the thymus. Semin Immunol 4: 363-369. [Crossref]
- Drachman DB (1994) Myasthenia gravis. N Engl J Med 330: 1797-1810. [Crossref]
- Willcox N. The thymus in myasthenia gravis patients, and the in vivo effects of corticosteroids on its cellularity, histology and functions. In: Kendall MD, Ritter MA, editors. Thymus update. Har-wood Academic Publ, Amsterdam 1989, p 105–24.
- Berrih S, Safar D, Levasseur P, Gaud C, Bach JF (1984) The in vivo effects of corticosteroids on thymocyte subsets in myasthenia gravis. J Clin Immunol 4: 92-97. [Crossref]
- Fattorossi A, Battaglia A, Buzzonetti A, Minicuci G, Riso R, et al. (2008) Thy-mopoiesis, regulatory T cells, and TCRVbeta expression in thymoma with and without myasthenia gravis, and modulatory effects of steroid therapy. J Clin Immunol 28: 194-206.
- Nieto MA, González A, Gambón F, Díaz-Espada F, López-Rivas A (1992) Apoptosis in human thymocytes after treatment with glucocorticoids. Clin Exp Immunol 88: 341-344. [Crossref]
- Tiso M, Gangemi R, Bargellesi Severi A, Pizzolitto S, Fabbi M, et al. (1995) Spontaneous apoptosis in human thymocytes. Am J Pathol 147: 434-444. [Crossref]
- Steinmann GG (1986) Changes in the human thymus during aging. Curr Top Pathol 75: 43-88. [Crossref]
- George AJ, Ritter MA (1996) Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today 17: 267-272. [Crossref]
- Miracle X, Di Renzo GC, Stark A, Fanaroff A, Carbonell-Estrany X, et al. (2008) Guideline for the use of antenatal corticosteroids for fetal maturation. J Perinat Med 36: 191-196. [Crossref]
- Kuypers E, Collins JJ, Jellema RK (2012) Ovine fetal thymus response to lipopolysaccharide-induced chorioamnionitis and antenatal corticosteroids. PLoS One 7: e38257.
- Diepenbruck I, Much CC, Krumbholz A, Kolster M, Thieme R, et al. (2013) Effect of prenatal steroid treatment on the developing immune system. J Mol Med (Berl) 91: 1293-1302. [Crossref]
- Michie CA, Hasson N, Tulloh R (1998) The neonatal thymus and antenatal steroids. Arch Dis Child Fetal Neonatal Ed 79: F159. [Crossref]
- Kotiranta-Ainamo A, Apajasalo M, Pohjavuori M, Rautonen N, Rautonen J (1999) Mononuclear cell subpopulations in preterm and full-term neonates: independent effects of gestational age, neonatal infection, maternal pre-eclampsia, maternal betamethason therapy, and mode of delivery. Clin Exp Immunol 115: 309-314.
- Chabra S, Cottrill C, Rayens MK, Cross R, Lipke D, et al. (1998) Lymphocyte subsets in cord blood of preterm infants: effect of antenatal steroids. Biol Neonate 74: 200-207. [Crossref]
- Seddon B, Mason D (2000) The third function of the thymus. Immunol Today 21: 95-99. [Crossref]
- Stephens LA, Mottet C, Mason D, Powrie F (2001) Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol 31: 1247-1254. [Crossref]
- Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, et al. (2002) Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med 196: 379-387. [Crossref]
- Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M (2005) A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest 115: 1953-1962. [Crossref]
- Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, et al. (2006) Persistence of naive CD45RA+ regulatory T cells in adult life. Blood 107: 2830-2838. [Crossref]
- Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E (2002) Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology 106: 190-199. [Crossref]
- Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, et al. (2010) Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol 184: 4317-4326. [Crossref]
- Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, et al. (2006) Fetal thymic involution: a sonographic marker of the fetal inflammatory response syndrome. Am J Obstet Gynecol 194: 153-159.
- Yinon Y, Zalel Y, Weisz B, Mazaki-Tovi S, Sivan E, et al. (2007) Fetal thymus size as a predictor of chorioamnionitis in women with preterm premature rupture of membranes. Ultrasound Obstet Gynecol 29: 639-643. [Crossref]
- El-Haieg DO, Zidan AA, El-Nemr MM (2008) The relationship between sonographic fetal thymus size and the components of the systemic fetal inflammatory response syndrome in women with preterm prelabour rupture of membranes. Br J Obstet Gynaecol 115: 836-841.
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK (1985) Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. Am J Obstet Gynecol 151: 333-337. [Crossref]
- Zalel Y, Gamzu R, Mashiach S, Achiron R (2002) The development of the fetal thymus: an in utero sonographic evaluation. Prenat Diagn 22: 114-117. [Crossref]
- Cho JY, Min JY, Lee YH, McCrindle B, Hornberger LK, et al. (2007) Diameter of the normal fetal thymus on ultrasound. Ultrasound Obstet Gynecol 29: 634-638. [Crossref]
- Battaglia A, Buzzonetti A, Baranello C, Fanelli M, Fossati M, et al. (2013) Interleukin-21 (IL-21) synergizes with IL-2 to enhance T-cell receptor-induced human T-cell proliferation and counteracts IL-2/transforming growth factor-ß-induced regulatory T-cell development. Immunology 139: 109-120.
- Catzola V, Battaglia A, Buzzonetti A, Fossati M, Scuderi F, et al. (2013) Changes in regulatory T cells after rituximab in two patients with refractory myasthenia gravis. J Neurol 260: 2163-2165. [Crossref]
- Gamez F, De Leon-Luis J, Pintado P, Perez R, Robinson JN, et al. (2010) Fetal thymus size in uncomplicated twin and singleton pregnancies. Ultrasound Obstet Gynecol 36: 302-307.
- Tarbox JA, Keppel MP, Topcagic N, Mackin C, Ben Abdallah M, et al. (2014) Elevated double negative T cells in pediatric autoimmunity. J Clin Immunol 34: 594-599.
- Kabelitz D, Déchanet-Merville J2 (2015) Editorial: "Recent Advances in Gamma/Delta T Cell Biology: New Ligands, New Functions, and New Translational Perspectives". Front Immunol 6: 371. [Crossref]
- Steffens CM, Al-Harthi L, Shott S, Yogev R, Landay A (2000) Evaluation of thymopoiesis using T cell receptor excision circles (TRECs): differential correlation between adult and pediatric TRECs and naïve phenotypes. Clin Immunol 97: 95-101.
- Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Möwes B, et al. (2002) Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med 195: 789-794. [Crossref]
- Kohler S, Thiel A (2009) Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood 113: 769-774. [Crossref]
- Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, et al. (2006) Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A 103: 6659-6664.
- Popmihajlov Z, Smith KA (2008) Negative feedback regulation of T cells via interleukin-2 and FOXP3 reciprocity. PLoS One 3: e1581. [Crossref]
- Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, et al. (2007) Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 19: 345-354. [Crossref]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, et al. (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30: 899-911. [Crossref]
- Berry LM, Polk DH, Ikegami M, Jobe AH, Padbury JF, et al. (1997) Preterm newborn lamb renal and cardiovascular responses after fetal or maternal antenatal betamethasone. Am J Physiol 272: R1972-1979. [Crossref]
- Fukuhara K, Okumura M, Shiono H, Inoue M, Kadota Y, et al. (2002) A study on CD45 isoform expression during T-cell development and selection events in the human thymus. Hum Immunol 63: 394-404.
- Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, et al. (2005) Thymic regulatory T cells. Autoimmun Rev 4: 579-586. [Crossref]
- Pole JD, Mustard CA, To T, Beyene J, Allen AC (2009) Antenatal steroid therapy for fetal lung maturation: is there an association with childhood asthma? J Asthma 46: 47-52. [Crossref]
- Akdis CA, Akdis M (2015) Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J 8: 17. [Crossref]
fi


