Abstract
The developmental process of human antibody (Ab)-secreting B cells in humanized mice is not fully defined. To examine the characteristics of class-switched and Ab-secreting B cells in humanized mice, we analyzed NOD/Shi-scid, common γc-null mice reconstituted with cord blood-derived hematopoietic stem cells (CB-NOG). Most of the CD138-positive plasmablasts (PBs)/plasma cells (PCs) in the NOG spleen maintained CD5 expression. Moreover, IgG-bearing B cells, including PBs and memory cells, predominantly had a CD21-CD24hiCD5+ phenotype. We examined the expression of CD21, CD24 and CD5 on B cells developed in CB-NOG mice. We observed three types of B cells (CD24high CD21-; T1, CD24low/CD21-; T2, CD24-CD21+; T3) within CB-NOG CD5+ cells, which are similar to transitional B cells. However, the surface marker expression was different from normal transitional B cells. First, CD21 expression was significantly lower than in human peripheral blood and CB-derived mature B cells. Second, the expression of CD5 increased according to the developmental stage. The splenocytes were sorted according to the transitional subsets and examined for IL-10 expression. The CD21-CD24hiCD5+ fraction had the highest IL-10 expression among the CD5+ cells, suggesting the involvement of regulatory B cells. The same fractions were cultured in the presence of CpG and IgM. These stimulated B cells secreted a low level of IgM and IgG Abs to CpG and IgM stimulation in vitro and maintained a CD5+CD21low phenotype. These results demonstrate that IgG-bearing B cells with a CD21-CD24hiCD5+ irregular phenotype are developed in humanized NOG mice and have become the source of antibodies in CB-NOG mice. (250 words)
Key words
immunodeficient mouse, B cell, CD5, plasma cells, human cord blood cells, human transitional B cell, immunodeficient mouse, antibody secretion, hematopoiesis, transplantation
Abbreviations
Ab: antibody, BM: bone marrow, Breg: regulatory B cell, CB: cord blood, CSR: class switch recombination, DNP-KLH: dinitrophenyl-keyhole limpet hemocyanin, GC: germinal center, HSC: hematopoietic stem cell, MFI: mean fluorescent intensity, MNC: mononuclear cell, MZ: marginal zone, NOG: NOD/SCID/IL-2gc-null, PB: peripheral blood, T1/T2/T3 cell: transitional 1/2/3 B cell, TLR: Toll-like receptor, TSST-1: toxic shock syndrome toxin-1.
Introduction
A humanized mouse immune system is established by transplanting CD34+ cells from human cord blood into severely immunodeficient NOG mice (CB-NOG). It has been shown that human T cells and B cells can develop in these mice, although the cells are only partially functional in xenograft environments [1,2]. The B cells of these mice can secrete antigen-specific IgM, natural IgM and IgG Abs. However, the B cells cannot produce normal levels of antigen-specific Abs [1,3,4]. We hypothesize that the development of B cells is not fully reconstituted and that B cells with an irregular transitional phenotype directly differentiate into plasma cells to secrete IgM and IgG antibodies in CB-NOG mice.
There are reports of the analyses of B cells developed in CB-NOG mice, which demonstrate that the mature B cells are reduced compared to normal mice. Moreover, while the B cells secrete natural IgM and IgG, antigen-specific IgG secretion is severely suppressed. At first, the reason for this reduction was thought to be a mismatch of the TCR and MHC because the TCRs on human T cells are selected by mouse MHC in the mouse thymus. However, HLA expression was not able to drive sufficient antigen-specific IgG secretion [5]
We speculated that the B cells may not mature from naïve B cells to normal plasma cells (PCs) in the humanized NOG environment because the supporting non-hematopoietic cells, such as follicular dendritic cells and dendritic cells, are absent in mouse lymph nodes and spleens and most of the B cells express CD5, which is known as a suppressive signal-inducing molecule [6]. These B cells are reported to contain a subset of regulatory B10 cells, which secrete IL-10 and might be induced by CD5 signals [7,8]. Although the developmental pathway of regulatory B cells has not been clarified, an irregular lymphopenic environment such as the humanized NOG mouse may induce regulatory B cell development, as in humans, patients who are lymphopenic following irradiation produce CD5+ B cells extensively [8].
Recently, Azulay-Debby et al. reported that immature B cells express Toll-like receptor 9 (TLR9) and that CpG stimulation induces IgM secretion [9]. Moreover, TLR9 signaling induces CSR in immature B cells and stimulates the production of IgG in immature B cells [10]. Transitional B cells isolated from human cord blood also produce IgG antibody after TLR9 stimulation [11]. After stimulation, these cells acquired a marginal zone (MZ) B cell phenotype. Thus, TLR9 signaling may induce antibody secretion irrespective of the activated stage of the B cells.
In the current study, we examined the B cells that secrete natural IgM and IgG Abs in humanized NOG mice. We found that most of the CB-NOG B cells that secrete Abs have a CD21-CD24+CD5+ irregular phenotype and secrete a large amount of IL-10.
Materials and methods
Mice
NOD/Shi-scid, common γc-null (NOD/SCID/γc-null; NOG) mice were provided by the Central Institute for Experimental Animals (Central Institute for Experimental Animals, Kawasaki, Japan). BALB/c mice were purchased from Crea Japan, Inc. (Kawasaki, Japan). All of the mice were housed in specific pathogen-free conditions in the animal facility located at the Tokai University School of Medicine (Isehara, Japan). All of the experiments were in compliance with the Guidelines of Care and Use of Laboratory animals and were approved by the committee of the Tokai University School of Medicine.
Human hematopoietic stem cells
Human umbilical cord blood (CB) was obtained from full-term, healthy newborns immediately after vaginal delivery. Informed consent was obtained according to the Institute guidelines and this research was approved by Tokai University Human Research Committee. MNCs were separated by Ficoll-Paque gradient centrifugation. CD34+ cells were purified from MNCs using a 2-step MACS purification method (Miltenyi Biotec, Gladbach, Germany), and the purity was greater than 98%.
Transplantation
Nine-week-old NOG mice were irradiated sublethally with 2.5 Gy prior to transplantation. The CD34+ cells were transplanted intravenously. PB was collected via retro-orbital bleeding under inhalation anesthesia eight weeks after the transplantation. MNCs were prepared and the reconstitution rates were calculated by hCD45 expression using FACS analysis. The mice were sacrificed and analyzed for B cell development after 8, 12 and 14 weeks.
Peptides
The Her-2 peptide includes the sequence identified as the epitope of an apoptotic anti-Her-2 Ab, CH401. The peptide was determined using MAP peptides with a partial amino acid sequence of Her-2/neu [12]. The sequence of the peptide is N:163-182 ((YQDTILWKDIFHKNNQLALT-BBB)-K4K2KB). This peptide was synthesized using a Rink amide resin (0.4-0.7 mmol/g) and an ACT357 peptide synthesizer (Advanced Chemtech, Louisville, KY). Each of these MAP peptides was coated on a 96-well plate, and the cross-reactivity with CH401 was examined using an ELISA as previously described.
Monoclonal Abs, flow cytometry and cell sorting
The cells were analyzed using a FACSFortessa or FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA). Cell sorting was performed using a FACSAria (Becton Dickinson). The cells were incubated with appropriately diluted, fluorescently labeled primary mAbs for 15 min at 4°C and were then washed with PBS containing 1% (w/v) BSA. For each analysis or sorting, the live gate containing white blood cells or lymphocytes was further gated on human CD45+ cells. The mouse anti-human monoclonal Abs (mAbs) used in this study are listed in Table S1.
Supplementary Table-1
CD |
FL |
clone# |
Company |
CD1d |
PE |
51.1 |
eBioscience |
CD3 |
PerCP-cy5.5 |
SP34-2 |
BD Pharmingen |
CD4 |
APC |
SK3 |
BD Biosciences |
CD5 |
PE-cy7 |
UCHT2 |
Biolegend |
CD8 |
FITC |
HIT8a |
BD Biosciences |
CD10 |
PE |
HI10a |
BD Pharmingen |
CD11c |
APC |
3.9 |
eBioscience |
CD14 |
FITC |
61D3 |
eBioscience |
CD19 |
APC-cy7 |
HIB19 |
Biolegend |
CD21 |
APC |
544408 |
RD |
CD24 |
PerCP-cy5.5 |
ML5 |
Biolegend |
CD25 |
APC-cy7 |
BC96 |
Biolegend |
CD27 |
PE |
M-T271 |
BD Biosciences |
CD34 |
FITC |
581 |
BD Pharmingen |
CD38 |
alexa fluor700 |
HIT2 |
Biolegend |
CD40 |
PE |
5C3 |
eBioscience |
CD45 |
Pacific Blue |
HI30 |
Biolegend |
CD117 |
APC |
YB5.B8 |
BD Pharmingen |
IgM |
PE |
G18-145 |
BD Pharmingen |
IgD |
FITC |
IA6-2 |
BD Pharmingen |
Stimulation of B cells
CB-NOG bone marrow cells and spleen cells were prepared and specific cell fractions were sorted as described in the above section. The cells were resuspended at 2 × 105/ml and cultured for 48 hours in RPMI 1640 medium supplemented with 10% FBS in the presence of 10 μg/ml LPS (Escherichia coli serotype 0111, Sigma) in 96-well flat-bottom plates.
The sorted B cell fractions were seeded into 96-well flat-bottom plates and stimulated with anti-IgM mAb (Beckman Coulter, CA, USA, 0.02 µg/ml) with CpG (Cosmo Bio, Tokyo JPN, 1.5 nmol/ml) in vitro. The supernatants and cells were collected and the surface markers and secreted antibodies were analyzed by ELISA or flow cytometry seven days after stimulation.
RT-PCR
RNA was extracted from the stimulated cells using an RNeasy Mini Kit (Qiagen, Germantown, MD). The total RNA (50 ng) was amplified using primers and the OneStep RT-PCR kit (Qiagen). The RT-PCR conditions were as follows: pre-incubation at 50°C for 30 min and polymerase activation at 95°C for 15 min, followed by 33 cycles of PCR. Each PCR cycle consisted of denaturation at 94°C for 1 min, annealing at 60°C for 1 min and extension at 72°C for 1 min. The PCR products were subjected to agarose gel electrophoresis. The following primers were used in this study: IL-10 Forward: 5’-CTTCGAGATCTCCGAGATGCCTTC-3’, Reverse: 5’-ATTCTTCACCTGCTCCACGGCCTT-3’; GAPDH Forward: 5’-GCCACCCAGAAGACTGTGGATGGC-3’, Reverse: 5’-CATGTAGGCCATGAGGTCCACCAC-3’. The RT-PCR products were subjected to agarose gel electrophoresis.
Real time PCR
The expression levels of mRNA for a housekeeping gene and IL-10 were measured by qPCR using the Bio-Rad CFX96 system (Bio-Rad, Hercules, CA, USA). GAPDH and IL-10 specific primers were purchased from Takara Bio (Otsu, Japan). The following primers were used: GAPDH (forward:5′- GCACCGTCAAGGCTGAGAAC-3’, reverse:5’- ATGGTGGTGAAGACGCCAGT-3’), and IL-10 (forward:5’- GAGATGCCTTCAGCAGAGTGAAGA-3’, reverse:5’- AAGGCTTGGCAACCCAGGTA-3’). Freshly isolated total RNA from the B cell fractions of CB-NOG mice was converted to cDNA using the PrimeScript™ RT Reagent Kit (Takara Bio) according to the manufacturer’s instructions. The PCR reaction consisted of 5 µl of SsoFast™ EvaGreen® Supermix (Bio-Rad), 3.5 µl of RNase/DNase free water, 0.5 µl of 5 µM primer mix, and 1 µl of cDNA in a final volume of 10 µl. The cycling conditions were as follows: 30 s at 95°C followed by 45 cycles of 1 s at 95°C and 5 s at 60°C. Melting curve analyses were performed from 65°C to 95°C at the end of each reaction to confirm the homogeneity of the PCR products. All assays were repeated three times and the mean values were used to determine the gene expression levels. We used five controls of 10-fold serial dilutions for each standard transcript to determine the absolute quantification, specificity and amplification efficiency of each primer set. Standard transcripts were generated by in vitro transcription of the corresponding PCR product into a plasmid. The nucleotide sequences were confirmed by DNA sequencing using the CEQ8000 Genetic Analysis System (Beckman Coulter, Fullerton, CA, USA). The sequence quality and concentration were validated using the Agilent DNA 7500 Kit in an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). GAPDH gene expression was used as an internal control. The expression levels of each target gene were normalized to GAPDH expression.
ELISA
ELISA was performed as described previously [3]. Briefly, microwells were coated with anti-human Igs (1:3000 v/v) (ICN, Aurora, OH, USA) diluted in carbonate buffer (pH 9.5) and adsorbed to microtiter plates (Sumiron, Tokyo, Japan) overnight at 4℃. The wells were washed with PBS-Tween (0.05% v/v) and blocked with 3% BSA-PBS at room temperature for two hours. After three washes with PBS-Tween, 10-fold serial dilutions of mouse serum or human IgM or IgG standard protein were added to the wells. The plates were then incubated for two hours at room temperature. The plates were washed three times before biotin-conjugated mouse anti-human IgM monoclonal antibody (mAb; Pharmingen, San Diego, CA, USA) (1:3000 v/v) or anti-human IgG mAb (Pharmingen) (1:3000) were added. After a 2-hour incubation at 37°C, the plates were washed three times and streptavidin-horseradish peroxidase (1:50,000 v/v; Pharmingen) was added. The plates were incubated for 1 hour at room temperature and the unbound conjugates were removed by washing. TMB peroxidase was then added. EIA substrate kit solution (Bio-Rad Laboratories, Hercules, CA, USA) was added to each well. The reaction was stopped with 10% HCl and the absorbance was measured at 450 nm.
Statistics
Statistical analysis was performed with Microsoft Excel (Microsoft, Redmond, WA. USA). The data shown are the mean ± SD. Significant differences between groups were determined by two-sided Student’s t-test analysis.
Results
CD138 and IgG-positive plasma cells have an irregular phenotype
To clarify which type of B cells secrete class-switched IgG antibodies, we first examined the surface markers of CD138+ cells in the NOG bone marrow and spleen. As antibody secreting plasma cells express CD138 and CD38, we analyzed the surface markers of CD138+CD19+ B cells in CB-NOG mice. As a result, more than 80% of CD138+CD19+ cells were CD5+, and the remaining CD38+ cells expressed a low level of CD5, suggesting that the B cells did not have a conventional B2 cell origin (Figure 1). Similar results were obtained for the BM CD138+ cells.
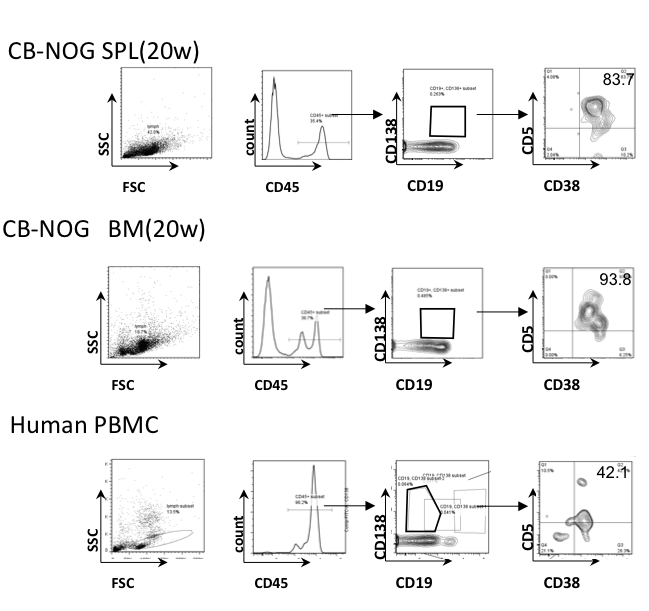
Figure 1.
Next, we examined whether IgG-positive memory cells possess a mature phenotype with low CD24 and/or high CD21 expression. Only 10% of the IgG+CD5+ cells in the spleen had a CD21high mature phenotype, while approximately 20% of the IgM+CD5+ B cells in spleen were CD21high, suggesting that most of the splenic B cells possess pre-mature or exhausted B cell characteristics with respect to CD5 expression (Figure 2). A fraction of the B cells were CD21/CD24 double-positive, a normal memory B cell marker expression pattern. On the other hand, a significant amount of IgG-bearing CD5lo cells were CD21loCD24high B cells, which suggests that more immature B cells went through class-switching in the periphery to become memory B cells. In contrast to the spleen cells, BM and PB B cells did not contain a significant amount of CD21high IgG or IgM-bearing B cells, suggesting that the mature B cells are not contained in these fractions or that the mature cells became exhausted.
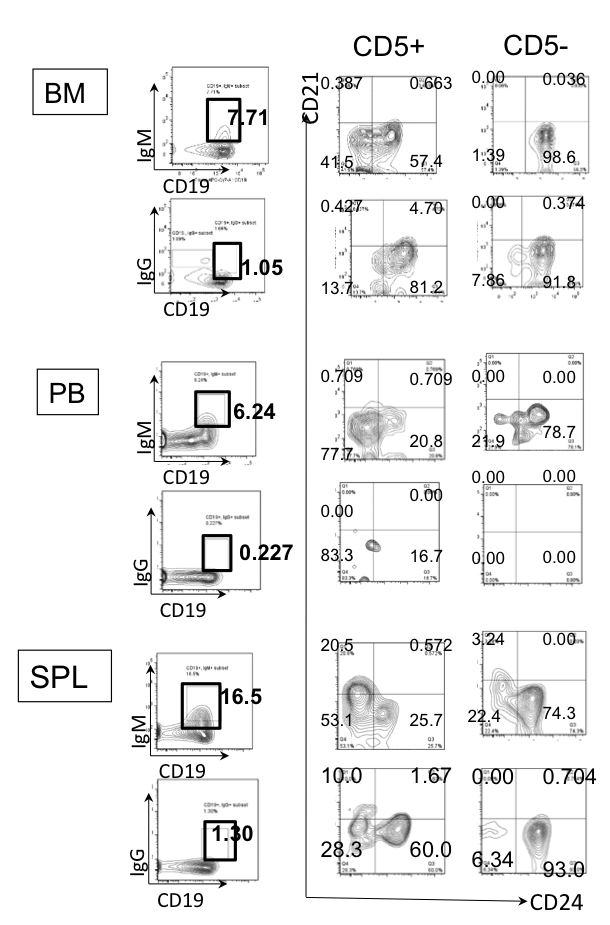
Figure 2. Phenotype of IgM/IgG-bearing cells in CB-NOG mice. CB-NOG B cells obtained from the BM, PB and spleen were stained with Mabs for human CD45, IgM, IgG, CD5, CD19, CD21 and CD24. hCD45+ cells were further gated for IgM-expressing cells and IgG-expressing cells. The expression of CD21 and CD24 was analyzed. Representative data from 5 mice were shown.
These results suggest that the IgG-bearing plasma/plasmablast cells in CB-NOG mice have a CD5+ B cell phenotype and the IgG bearing memory B cells possess an immature/exhausted phenotype.
Innate B cell subsets in CB-NOG mice
As CD138+ cells are all CD5+ in CB-NOG mice, we examined whether innate CD5-positive cells are extensively developed into plasma cells in the mice. The detailed developmental pathways of human innate B cells have not been elucidated, although they are thought to occur in different lineages than conventional B2 cells. Some of these B cells are reported to express CD5 in the mature stage or during developmental stages [11,13]. Thus, it is necessary to examine whether the CD5+ B cells found in CB-NOG mice contain B cell subsets such as B1a, MZ and Breg cells.
We analyzed whether CB-NOG mice could develop B1a cells and MZ B cells by staining the cells for IgD and CD27. B1a cells are included in CD5+/IgD-/CD27+ cells and MZ cells are included within CD5+IgD+CD27+ cells. Therefore, we can distinguish B1a and MZ cells from immature and transitional B cells [14]. As shown in Figure 3, a significant amount of CD5+/IgD-/CD27+ cells and CD5+/IgD+/CD27+ cells were detected in the CB-NOG BM and spleen. However, the ratio was significantly reduced in the total CD5+ cells, and most of the CD5+ cells were CD27-. This result suggests that most of the CD5+ B cells were neither B1 cells nor MZ B cells.
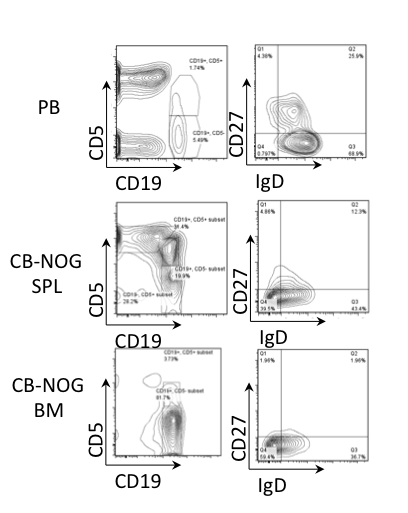
Figure 3. Innate B cell subsets do not accumulate in CB-NOG mice. PB and CB-NOG MNCs were stained for CD45, CD19, CD5, CD27 and IgD (upper panels, PB; middle panels, CB-NOG SPL; lower panels, CB-NOG BM). Lymphoid and hCD45-gated cells were further gated using CD19 and CD5 expression. They were further subdivided based on IgD and CD27 expression. As indicated, CD19+/CD5+/CD27+/IgD- cells are human B1a cells. Representative data from six independent experiments (n=6) are shown.
Collectively, our data show that most of the CD5+ B cells in CB-NOG mice are not normal innate B cells such as B1a or MZ B cells.
Differentiation markers to distinguish immature transitional naïve B cell stages
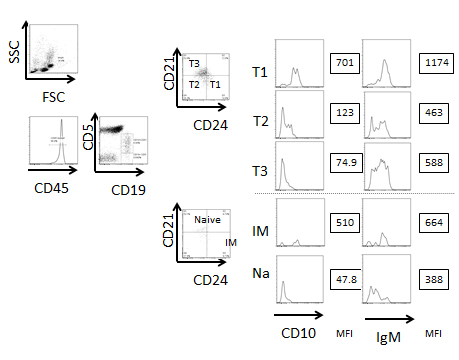
To determine the origin of the CD5+ B cells, we next analyzed the CD27-CD5+ B cells, which are categorized as transitional B cells. Conventional B2 cell differentiation from immature to naive mature B cells is divided into several stages. As shown in Figure 4, immature B cells express CD5 to become transitional 1 (T1) B cells before migrating into the spleen. These transitional B cells change their expression markers as they pass through the transitional 2 (T2) and transitional 3 (T3) stages. The cells then become conventional naïve B2 B cells. Because developmental markers for these transitions have not been standardized, we first analyzed the markers of human PB- and CB-derived B cells from healthy donors and defined the transitional stages. According to Suryani et al. [15], CD5, IgM, IgD, CD21, CD24, CD27 and CD38 are markers that detect the stages of B cell maturation (Figure 4). In both the PB and CB, the majority of the B cells were CD19+CD27- non-memory B cells (Figure 4). In the CB, we subdivided CD5+ cells into CD21-CD24+ (T1), CD21-CD24- (T2) and CD21+CD24+/-(T3) subsets. The CD5- cells were subdivided into CD21-CD24+ (immature) and CD21+CD24- (naïve) subsets. The expression of IgD was increased in the T2 to T3 fractions and CD38 was slightly decreased in these fractions. These results suggest that maturation proceeds throughout the T1 to T3 transitional stages. CD10 was decreased in T2 and T3 cells and IgM expression was decreased (Figure S1), which suggests that the transitional B cell subset can be defined by these markers. Most of the PB B cells from healthy donors were naïve B cells and T3 B cells. In the CB, there were very few memory B cells, and the frequency of transitional B cells was higher than in PB B cells. The cell percentage at each developmental stage is shown in Figure 4. Compared to the PB, immature and transitional B cells were more abundant within the CB MNCs. Collectively, these results show that these markers can distinguish immature, T1, T2, T3 and naïve B cells. Our subsequent analyses used these markers to identify B cell fractions.
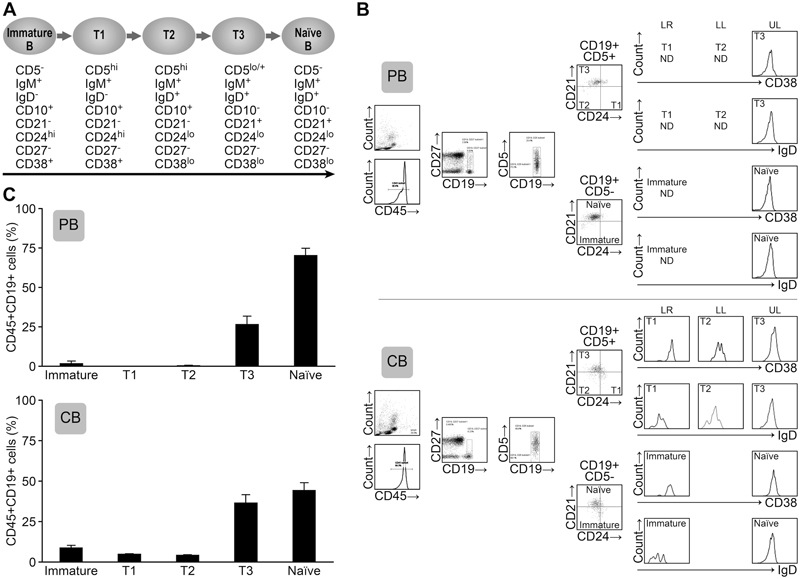
Figure 4. The B cells within PB and CB MNCs contain high frequencies of transitional B cells. A. The defined peripheral B cell stages and the markers are shown. B. The blood MNCs were stained with antibodies against CD45, CD19, CD5, IgD, CD38, CD27, CD24 and CD21 (Upper panels; PB, lower panels; CB). The lymphoid-gated cells were further gated for hCD45 expression. The hCD45+ cells were further gated for CD19+CD27- non-memory cells. Then, the cells were divided into CD19+/CD5+ and CD19+/CD5- cell fractions. These cells were further analyzed for CD21 and CD24 expression and defined as immature, transitional 1 (T1), T2, T3 and naïve B cells. (UL; upper left, LL; lower left, UR; upper right, LR; lower right) CD38 and IgD expression levels were also analyzed. The FACS plot is representative of six independent experiments. C. The subset cell percentages are shown as the frequency of CD45+CD19+CD27- cells. IM; immature B cell, T1; transitional 1 B cell, T2; transitional 2 B cell, T3; transitional 3 B cell, N; naïve B cell. Representative data from 4 experiments are shown. The mean percentage ± SD is shown (n=6).
CB-NOG-derived B cells accumulated at the transitional 3 stage in the spleen
We examined the CD5, CD19, CD21 and CD24 expression levels in addition to IgD and CD38 expression to clarify which B cells (i.e., immature, transitional or mature naive B cell stages) develop in CB NOG mice. As shown in Figure 5 and Figure S3, most of the BM B cells (96%) were immature B cells. However, in the spleens, immature and T2 cells were dominant and fewer T3 cells were present. The expression level of CD21 on the T3 B cell subset was significantly lower than normal human PB and CB B cells. The frequencies of immature cells and T2 cells in the PB were dominant and were significantly higher than in CB and PB MNCs (Figure 4). T3 stage B cells were not detectable in the CB-NOG PB. Collectively, CD21 expression was significantly lower in CB-NOG B cells.
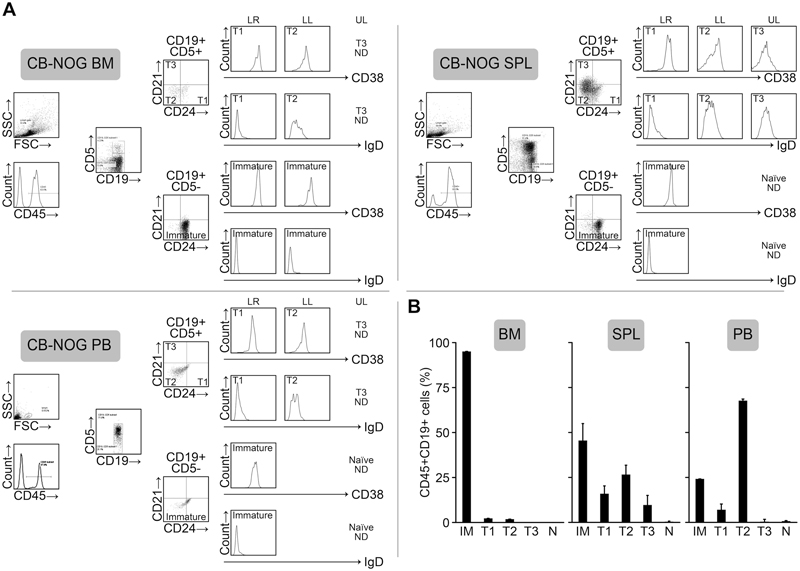
Figure 5. B cells that develop in the CB-NOG mouse contain B cells with CD21low expression. A. Bone marrow, spleen and PB B cells from NOG mice (14 weeks after transplantation) were stained for CD45, CD19, CD5, CD21, CD24, CD27, CD38 and IgD. Lymphoid and hCD45-gated CB-NOG BM (upper panels) and spleen (lower panels) MNCs were further gated according to CD19 and CD5 expression (very few CD27+ cells were detected, and we negated this fraction in the panel). These cells were further subdivided based on CD21 and CD24 expression. The expression of CD38 and IgD in each fraction was shown as histograms. (UL; upper left, LL; lower left, LR; lower right) B. The percentages of the subsets within lymphoid-gated fractions of hCD45+/CD19+ cells are shown. BM, spleen and PB MNCs from CB-NOG mice were prepared 14 weeks after transplantation. The mean percentage ± SD is shown (n=5).
Figure 5B shows the ratio of the B cell subsets in the CD45+/CD19+ cell subset (mean ± SD, n = 5, 14 weeks after the transplantation). These results demonstrate that most of the B cells in CB-NOG mice are CD21- immature and transitional/exhausted B cells in the spleen and in the PB.
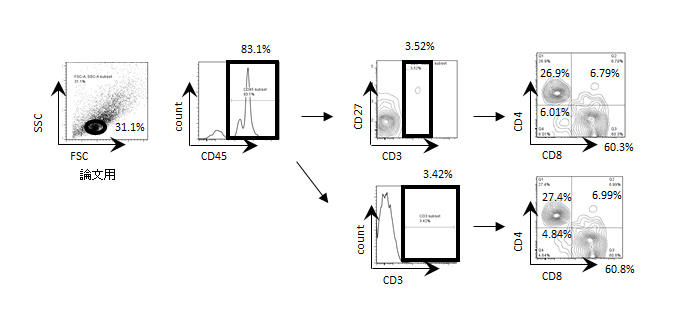
With respect to T cell development, we observed a few CD5+CD19- cells that include T cells at 14 weeks after transplantation. The mature Th and Tc cell development is consistent with data previously reported by our group and others (Figure S2).
CD5 expression in B cells with a transitional phenotype
As surface expression of CD5 is prominent in CB-NOG B cells, we compared the mean fluorescence intensity (MFI) of CD5 among transitional B cells and found that CD5 expression was highest in the T3 cells. This result suggests that in CB-NOG T3 B cells, surface CD5 expression is not decreased (Figure 7A).
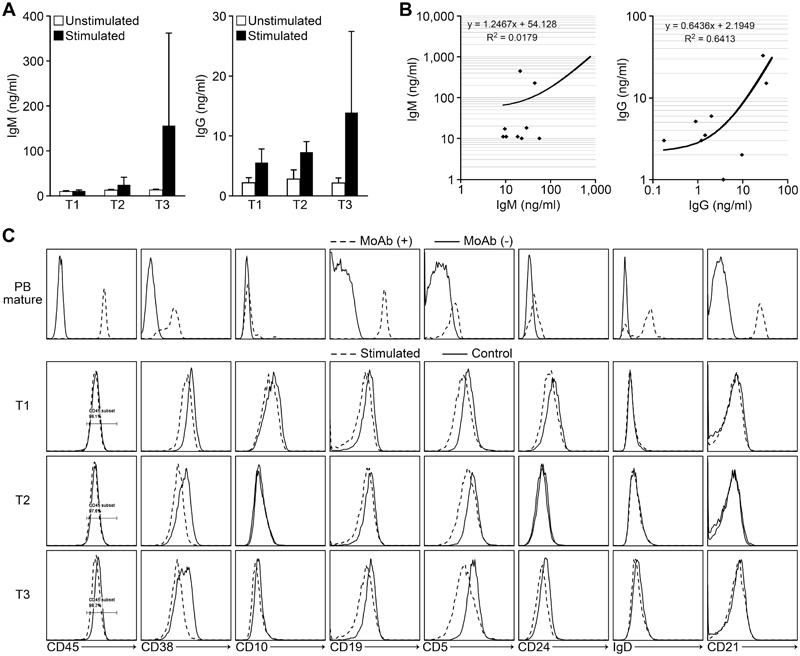
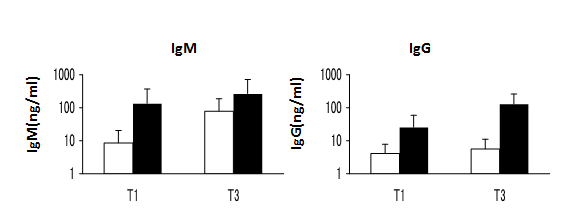
Figure 6. IgM and IgG secretion by transitional B cells that developed in CB-NOG mice. The sorted spleen B cells were greater than 90% pure. The cells were stimulated with IgM and CpG in vitro. The supernatants were used to evaluate Ab secretion by ELISA and the results were compared with the serum Ab level. A. Upper panel: natural IgM secretion by sorted transitional B cells. Open bars: non-stimulated B cells. Closed bars: CpG and anti-IgM stimulated B cells. CTRL indicates the non-stimulated B cells (n=9). Lower panel: natural IgG secretion by the same cell fractions. B. Upper panel: the correlation between non-antigen-specific IgM secreted by sorted T3 B cells and natural IgM in the CB-NOG sera. Lower panel: the correlation between natural IgG secreted by sorted T3 B cells and natural IgG in the CB-NOG sera. C. Expression of B cell differentiation markers. PB mature B cells: solid lines, isotype control; broken lines, Abs. The PB mature B cells, including memory B cells as the CD27-positive cells, were not gated. CB-NOG transitional B cells (T1, T2 and T3) seven days after the stimulation: solid lines, non-stimulated B cells. Broken lines indicate the cells after seven days of stimulation. Representative data from six experiments were shown.

Figure 7. A. Representative CD5 expression in immature, T1, T2 and T3 B cells is shown. The cells were gated for IM, T1, T2 and T3 subsets, as shown in Figure 4. The gated cells were analyzed for CD5 expression. Representative data from 10 mice are shown. B. Immature, T1 and T2/T3 cells were purified from the BM and spleen cell fractions 12 weeks after the transplantation using a FACSAria to greater than 90% purity. Sorted cell fractions were stimulated with LPS and the total RNA was submitted for RT-PCR. For real time PCR, T1, T2 and T3 cells were purified. GAPDH was used as the positive control. SP, total spleen MNCs; IM, immature B cells; T1, T2, T3, transitional B cell subsets, HD; healthy donor. The representative data from three independent experiments (n=3 for RT-PCR) are shown.
Recently, regulatory cell subsets called Breg or B10 cells have been described. These B cells secrete large amounts of IL-10 and regulate immune responses. In mice, these cells express CD5 [7], and the majority of human Breg cells also express CD5 [16]. We examined whether high levels of IL-10 were produced by the immature, T1 and T2/3 cell fractions that developed in CB-NOG mice. The immature T1 and T2/T3 fractions were sorted from CB-NOG B cells according to expression of surface markers. The expression of IL-10 was examined using RT-PCR analysis. IL-10 mRNA was detected in 12-week-old CB-NOG immature BM cells and spleen T1 cells (Figure 7B). These results suggest that immature CD24hiCD38hi cells can secrete IL-10 and may involve Breg subsets.
Transitional B cells from NOG mice can differentiate to secrete IgM and IgG Abs with and without CpG stimulation
CB-NOG mice produced IgM and IgG after reconstitution of human T cells and B cells without any stimulation. Thus, we examined whether the irregular B cells secrete these antibodies. Splenic transitional B cells (CD45+CD19+CD27-CD5+) from CB-NOG mice were sorted into T1, T2 and T3 fractions according to CD21 and CD24 expression. After stimulation with CpG and anti-human IgM, the culture supernatants and cells were collected and assayed by ELISA [17] and FACS analysis, respectively. As shown in Figure 6, stimulated and non-stimulated B cell fractions secreted IgM and IgG irrespective of their stages. T3 B cells tended to secrete more antibodies compared to the other two stages, but no significant difference was observed. The unstimulated cells also secreted significant levels of antibodies. These results suggest that spontaneous Ig-secreting B cells or memory B cells exist within the splenic B cell fractions. The IgG secretion of T3 cells induced by CpG/IgM stimulation in vitro was positively correlated with the level of total IgG in the CB-NOG plasma (r2=0.6413), while a very low correlation was observed between the IgM level secreted by T3 cells after CpG/IgM stimulation and IgM levels in the CB-NOG plasma (r2=0.0179) (Figure 6). These results suggest that the major source of serum IgG was provided by stimulated irregular B cells in vivo. Furthermore, the major source of IgM is not developed from transitional B cells and may be the CD5+CD138+ subsets shown in Figure 1 that secrete IgM. After stimulation, the expression of CD5 and CD38 B cell markers was slightly decreased in all three stages, but the other markers were not changed (Figure 6C). The CD38 levels were comparable with PB mature B cells in the T3 stage. However, the CD5 expression level was not as low as in mature B cells. Moreover, the expression levels of CD21 and IgD were lower than the expression on mature B cells. Therefore, most of the B cells were considered to be in an immature stage. The CD10 expression of these cells was very low in the T2 and T3 stages. This result suggests that these B cells may be exhausted B cells, which cannot express a normal amount of CD21. However, other maturation processes were intact and class-switch was possible. As CB-derived T3 B cells also secrete IgM and IgG (Figure S3), antibody secretion by some plasma cells with a transitional/exhausted B cell phenotype may not be an artifact of the xenograft environment in humanized NOG mice, and it is a normally observed phenomenon. These results demonstrate that B cells with a transitional/exhausted phenotype can secrete antibodies, including IgM and IgG, in CB-NOG spleens without expressing high levels of CD21.
Discussion
It is well known that the NOG mouse can develop human T cells and B cells from human HSCs in vivo and produce natural IgM and IgG. However, the B cells in these mice scarcely produce antigen-specific IgG. The reason was thought to be the mismatch of human TCR and human HLA because human T cells are restricted to mouse MHC. However, HLA expression could not completely recover the immune reaction with specific antibody secretion. We hypothesized that the B cells cannot develop into normal mature B2 cells and secrete antigen-specific class-switched Igs. Our results demonstrate that irregular CD5+ B cells are the main source of natural antibodies secreted in humanized NOG mice.
Although human CD5+ B cells have classically been categorized as B1a cells, they are now thought to include transitional and/or pre-naïve B cells [18-22]. We confirmed that the CD45+CD27-CD5+ cells are transitional B cells by comparing the expression of IgD and CD38 (Figure S3). We also assessed the expression of IgM, CD10, CD40 and CD23, and no differences from the transitional phenotype were observed (data not shown). Here, we found that CD138+ plasma cells expressed a high level of CD5 and a low level of CD21. Compared to the relatively normal human T cell development that occurs in the xenograft environment [2,4,23,24], human B cell development is complex in this mouse environment. Our results show that there are very few CD21high T3 cells in the CB-NOG PB, although these cells were found in the spleen. These results may suggest that immature to transitional T1 and T2 B cells develop in the BM or spleen and that CD21 starts to be expressed when cells are in the spleen. However, the CD21 expression level was very low, and these cells might not return to the circulation or survive in the circulation.
There are reports suggesting that the stimulation of B cells with CpG or IL-21 to increase BLIMP1 expression could stimulate the final differentiation of B cells to plasma cells [25,26]. In our system, stimulation with CpG and BCR crosslinking could not induce the phenotype of mature B cells, although the cells expressed the terminally differentiated plasma cell marker CD138. Although the memory B cell-specific marker CD27 is expressed on a subpopulation of the B cells in the CB-NOG mice, most of the CD27+ B cells were CD5+ cells. Thus, affinity maturation via GC reactions might not be induced in the CB-NOG mouse system, which suggests that other stimuli are needed in this mouse system to achieve naïve B2 B cell to plasma cell differentiation [27]. As we and others have previously reported, follicles and GCs rarely form in CB-NOG mice [1,28,29]. In this system, B cell maturation from the transitional to the naïve B cell stage may also be impeded. The impaired transition might be due to defects in the microenvironment [30-32].
It is also well accepted that CB-NOG mice secrete non-specific IgM and IgG as well as antigen-specific IgM Abs [32]. Our results first demonstrated that these Abs were also secreted by plasma cells with a unique B cell phenotype defined by the expression of CD21 and CD24. There are several reports demonstrating that Abs are secreted by immature and transitional B cells [15,33,34]. Mouse B10 cells have also been reported to secrete antigen-specific Abs and auto-Abs, which have a phenotype similar to transitional or immature B cells [35]. The functions of these antibodies are not clear, but they are thought to have immunoregulatory function. The CB-NOG T cell response is slightly inhibited compared to the normal response, and the existence of Breg cells or innate IgM may cause the low reactivity of CB-NOG T cells [36]. The T1 cells are CD38high and secrete high levels of IL-10. Thus, they may include Breg cells [7], as shown in Figure 3C. The Breg cells themselves may be an immature/T1 cells, which eventually suppress T cell function.
Compared to T1 and T3 cells, T2 cells are unique due to their high frequency in the PB of NOG mice. The characteristics of T2 cells are similar to so-called “exhausted” B cells, and they may be exhausted, non-reactive, anergic B cells. In CB-NOG mice, such non-reactive B cells may accumulate in the periphery. CD21 is a CR2 receptor that is involved in the co-signaling complex consisting of the B cell receptor together with CD19 and CD81. The co-signal is known to be induced by follicular dendritic cells (FDCs), but CB-NOG mice cannot develop FDCs. This result suggests that the co-signal may not be induced in this mouse system. As CD19-deficient mice cannot induce efficient reactions [37], specific IgG may not be induced in the FDC-deficient NOG environment in our system.
Our data show that non-specific IgG was secreted by stimulated and non-stimulated CD5+ B cells, and the amount was highly correlated with the serum IgG level. These results suggest that the main source of natural IgG Abs is CD5+ B cells, which have a very similar phenotype to transitional B cells. We also found that IgG-bearing cells display a transitional phenotype. In human cord blood, the transitional B cells can be developed into PCs directly to secrete both IgM and IgG, as reported previously (Figure S3) [10]. AID is expressed in the immature stage, which suggests that it is possible for immature B cells to produce IgG Abs. Thus, our finding may not be a xenograft phenomenon and might be a common characteristic of transitional B cells. Recently, TLR7-transgenic mouse T1 cells were reported to be activated and to secrete IgM and IgG from endogenous RNA [34]. The antibodies detected in CB-NOG mice might be produced partially via similar mechanisms. Alternatively, chronic stimulation of B cells with endogenous virus, bacteria or auto-antigens may induce the abnormal maturation of transitional B cells to produce natural Ab.
The abnormal accumulation of transitional CD5+ B cells suggests that there is a disturbance in the early stages of B cell homeostasis. This result has been reported in systemic lupus and Sjogren’s syndrome, but the contribution of the B cell population to these diseases is not clear [19,38]. An increase in auto-Abs and short-lived plasma cells has been reported in these autoimmune diseases, and the transitional B cell population may contribute to the supply of the Abs or the PCs. CB-NOG mice may provide a suitable system to clarify the B cell fate, including the initiation of Ab secretion and CSR and the development of the cells into short-lived PBs/PCs.
In summary, most of the human B cells that develop in CB-NOG mice are in the CD5+ transitional stage, and natural IgM and IgG Abs are secreted by these CD5+ B cells without differentiating into the CD5- phenotype. This mouse system may allow the investigation of the peripheral expression of CD5 on B cells.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (ITO) (S) [grant number 22220007]; a Tokai University grant Grant-in-aid Aid to K.Y. (2013-2014); and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (2012-2016).
Acknowledgements
We thank Dr. Kitamura from the Tokyo University of Science for reading our manuscript and providing suggestions. We also thank Yumiko Nakagawa for her excellent animal care skills. We thank the members of the Teaching and Research Support Center in Tokai University School of Medicine for their technical skills.
References
- Matumura T, Kametani Y, Ando K, Hirano Y, Katano I, et al. (2003) Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gcnull (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp Hematol 31: 789-797. [Crossref]
- Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, et al. (2002) Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J Immunol 169: 204-209. [Crossref]
- Kametani Y, Shiina M, Katano I, Ito R, Ando K, et al. (2006) Development of human-human hybridoma from anti-Her-2 peptide-producing B cells in immunized NOG mouse. Exp Hematol 34: 1240-1248. [Crossref]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, et al. (2005) Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 106: 1565-1573. [Crossref]
- Akkina R (2013) Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol 25: 403-409. [Crossref]
- Soldevila G, Raman C, Lozano F (2011) The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr Opin Immunol 23: 310-318. [Crossref]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, et al. (2008) A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28: 639-650. [Crossref]
- Youinou P, Jamin C, Lydyard PM (1999) CD5 expression in human B-cell populations. Immunol Today 20: 312-316. [Crossref]
- Azulay-Debby H, Edry E, Melamed D (2007) CpG DNA stimulates autoreactive immature B cells in the bone marrow. Eur J Immunol 37: 1463-1475. [Crossref]
- Edry E, Azulay-Debby H, Melamed D (2008) TOLL-like receptor ligands stimulate aberrant class switch recombination in early B cell precursors. Int Immunol 20: 1575-1585. [Crossref]
- Guerrier T, Youinou P, Pers JO, Jamin C (2012) TLR9 drives the development of transitional B cells towards the marginal zone pathway and promotes autoimmunity. J Autoimmun 39: 173-179. [Crossref]
- Miyako H, Kametani Y, Katano I, Ito R, Tsuda B, et al. (2011) Antitumor effect of new HER2 peptide vaccination based on B cell epitope. Anticancer Res 31: 3361-3368. [Crossref]
- Bouaziz JD, Yanaba K, Tedder TF (2008) Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev 224: 201-214. [Crossref]
- Griffin D, Holodick N, Rothstein T (2011) Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70-. J Exp Med 208: 67-80.
- Suryani S, Fulcher D, Santner-Nanan B, Nanan R, Wong M, et al. (2010) Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood 115: 519-529.
- Blair P, Noreña L, Flores-Borja F, Rawlings D, Isenberg D, et al. (2010) CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32: 129-140.
- Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, et al. (2004) Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 103: 2058-3064.
- Marie-Cardine A, Divay F, Dutot I, Green A, Perdrix A, et al. (2008) Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clinical Immunol 127: 14-25.[Crossref]
- Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE (2009) Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol 182: 4116-4126. [Crossref]
- Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, et al. (2009) Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol 182: 5982-5993. [Crossref]
- Jourdan M, Caraux A, Caron G, Robert N, Fiol G, et al. (2011) Characterization of a Transitional Preplasmablast Population in the Process of Human B Cell to Plasma Cell Differentiation. J Immunol 187: 3931-3941. [Crossref]
- Baumgarth N (2011) The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11: 34-46. [Crossref]
- Saito Y, Kametani Y, Hozumi K, Mochida N, Ando K, et al. (2002) The in vivo development of human T cells from CD34(+) cells in the murine thymic environment. Int Immunol 14: 1113-1124. [Crossref]
- Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, et al. (2003) Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood 102: 873-880. [Crossref]
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, et al. (2005) IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 175: 7867-7879. [Crossref]
- Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, et al. (2008) CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol 180: 800-808. [Crossref]
- Ito R, Shiina M, Saito Y, Tokuda Y, Kametani Y, et al. (2008) Antigen-specific antibody production of human B cells in NOG mice reconstituted with the human immune system. Curr Top Microbiol Immunol 324: 95-107. [Crossref]
- Watanabe S, Terashima K, Ohta S, Horibata S, Yajima M, et al. (2007) Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109: 212-218.
- Ito M, Kobayashi K, Nakahata T (2008) NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr Top Microbiol Immunol 324: 53-76. [Crossref]
- Shapiro-Shelef M, Calame K (2005) Regulation of plasma-cell development. Nat Rev Immunol 5: 230-242. [Crossref]
- Steiniger B, Ulfig N, Risse M, Barth PJ (2007) Fetal and early post-natal development of the human spleen: from primordial arterial B cell lobules to a non-segmented organ. Histochem Cell Biol 128: 205-215. [Crossref]
- Pereira JP, Kelly LM, Cyster JG (2010) Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol 22: 413-419. [Crossref]
- Ueda Y, Liao D, Yang K, Patel A, Kelsoe G (2007) T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol 178: 3593-3601.
- Giltiay NV, Chappell CP, Sun X, Kolhatkar N, Teal TH, et al. (2013) Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells. J Exp Med 210: 2773-2789. [Crossref]
- Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, et al. (2012) Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol 188: 1036-1048. [Crossref]
- Zhang X (2013) Regulatory functions of innate-like B cells. Cell Mol Immunol 10: 113-121. [Crossref]
- Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, et al. (2008) CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol 9: 63-72. [Crossref]
- Dörner T, Jacobi AM, Lipsky PE (2009) B cells in autoimmunity. Arthritis Res Ther 11: 247. [Crossref]