Introduction
Fibromyalgia syndrome (FMS) is a musculoskeletal pain and fatigue disorder with diffuse myalgia and lowered pain thresholds. Platelets they take up and store serotonin (5-HT) and when activated they release 5-HT. 5-HT re-uptake inhibitors prevent 5-HT uptake by the synapses, but these drugs are clinically less effective in FMS. The current study examined 5-HT content in density separated platelets.
Material and Methods
The study involves 20 female’s patients (age 39 ± 8 (SD) years) with FMS. 16 females (65 ± 8 years (SD) years) without FMS served as controls. The platelet population was separated according to density with a linear Percoll™ into 17 different density fractions. In all fractions, platelet counts was analyzed and determination of 5-HT content.
Results
The two study groups did not vary considerably concerning platelet distribution in the fractions. In contrast, normal and low-density platelets of FMS demonstrated higher serotonin content (p < 0.05).
Discussion
The study shows that platelets of FMS contain more serotonin. It is possible that platelets have additional storage locations of serotonin beside dense granules. The results may indicate that an abnormal sensitivity to pain may be due to an instability in the 5-HT system in FMS. Serotonin inhibitors, often given to FMS obstructs serotonin uptake in the synapses. It is therefore tenable that neurons eventually release a higher amount of serotonin. The circulating serotonin is taken up by platelets. As a result, there are to be a reduced effectiveness for these drugs in FMS, and patient will still encounter pain difficulties.
fibromyalgia, platelets, platelet heterogeneity, serotonin
Fibromyalgia syndrome (FMS)
FMS is considered as a persistent pain disease. FMS is more than often connected with extensive pain, several tender points, and low energy [1]. The victims are mostly women and occurs approximately 2% of the general population [2]. Anxiety, depressive symptoms and major depression and are a feature of the disease [3]. The origin of and pathophysiology of FMS is still uncertain. Pathophysiological theories contain modifications in detailed neurotransmitters such as serotonin (5-HT). A likely involvement of 5-HT in FMS could therefore be possible. Some evidence suggest the hypothesis that an insufficiency in serotonergic neuronal operation could be linked to the pathophysiology of FMS [4,5]. Low concentrations of 5-HT in cerebrospinal fluid and in serum have been discovered in FMS. It is hypothesized to have a meaning in the pain threshold [6].
Serotonin (5-HT)
It is well established that 5-HT is important in human physiology. Nearly, 90% of the body 5-HT substance is located in the upper gastrointestinal tract. The majority of circulating 5-HT is metabolized in the liver [7, 8]. 5-HT has a crucial role in synaptic activity additionally [9]. It influence cognitive functions for instance memory and learning [10]. Serotonergic neurons produce 5-HT in the central nervous system. Almost all brain areas express 5-HT receptors [10]. Platelets do not produce 5-HT [11] but they gather the molecule in a way similar to serotonergic neurons [12, 13]. The process needs energy [14, 15]. When activated 5-HT is released from platelet dense granules [11]. 5-HT2 receptors mediate the effect of 5-HT upon platelets [16]. 5-HT is a weak platelet agonist and does not activate platelets per se [17]. The activity of 5-HT is guarded by a particular 5-HT transporter (SETR), which arbitrates the intracellular reuptake of 5-HT. SETR are able to be blocked by discriminating 5-HT reuptake inhibitors (SSRIs). It is well known that platelets, central or peripheral serotonergic neurons and intestinal epithelial cells express SETR. Common molecular and physiological features are shared in these locations [18,19]. Opinion is divided whether SSRIs are effective or not at FMS. Nociception refers to the physiological process of transmitting a painful stimulus from the periphery through afferent neurons to the cerebral cortex. It has been hypothesized that serotonergic neurotransmission has an important purpose in nociception [20,21]; modifications in 5-HT metabolism and transmission could as a result be vital in the pathogenesis of FMS.
Platelet density
Platelets are able to differ in density by a span of 1.04–1.09 kg/l [22]. The most important deciding factor of density are platelet organelles i.e. platelets with high-density holds more α and dense granules compared to low-density cells [23]. A few researchers constitutes that platelet density increases as they get older [24,25], however an opposite view exists [26,27]. Some additional science describe that platelet density do not alter in the circulation [28-30]. The understanding of clinical understanding of platelet heterogeneity has been studied as well. Acute myocardial infarction is related with increasing platelet density [31]. Moreover, an opposite association exists among density and the inflammatory response in the latter disease [32]. Small high-density platelets are found in inflammatory bowel disease [33]. Essential thrombocythemia is typified by low peak platelet density [34].
Subjects
21 female patients with FMS, aged 39 ± 8 years (mean ± SD) took part in the study. All included FMS subjects were in search of care at the Pain and Rehabilitation Centre of the University Hospital, Linköping, Sweden. Patients completing the norm of the American College of Rheumatology standard for FMS were incorporated in the research (Wolfe et al). Clinical investigations and patient’s case histories established the clinical diagnoses of FMS. A control group (CON) consisted of 16 females aged 65 ± 8 years (mean + SD) were also involved in the study. All these subjects were free from FMS and they were all coming for a regular nurse led reception at a local health center. Every participants gave informed permission. The study was permitted by the community ethics board of Linköping University, Sweden (reg. number: 2012/269-32).
Laboratory investigations
All samples were taken in Vacutainer™ tubes (Becton and Dickinson, New Jersey, U.S.A.). Venous blood (7.5 ml) was anticoagulated with 2.5 ml 0.129 M disodium citrate. Platelets were separated according to density [35] by a linear Percoll™ gradient (GE Healthcare Bio-Sciences AB, Sweden). In order to fabricate Percoll™ gradient, two solutions (1.09 and 1.04 kg/L) were mixed as follows:
PercollTM solutions 1.09 (kg/L) 1.04 (kg/L)
PercollTM 32.84 (g) 8.88 (g)
H2O 11.42 (g) 19.14 (g)
Platelet-inhibitory solution 4.50 (g) 3.00 (g)
The platelet inhibitory solution was prepared by mixing following chemical substances:
1. 0.15 mol/L Na2 citrate* and 0.13 mol/L Na3EDTA* (pH 7.4 at 25oC).
2. 0.001 g/L prostaglandin E1* and 1 mL 95% ethanol in H2O.
3. 2.7 mmol/L theophylline* dissolved in 0.15 mol/L TRIS chloride buffer (pH 7.4 at 25oC).
* Sigma-Aldrich, Missouri, U.S.A.
In order to separate platelets according to density, following procedure was applied; first 7.63g of the 1.09 kg/L Percoll™ mixture was layered in the bottom of a 50 mL test tube. Thereafter 12.48 g of the 1.04 kg/L and 13.08 g of the 1.09 kg/L and Percoll™ solutions were employed into a two-chamber gradient maker. By this setting linear gradients were produced covering the density span 1.09 kg/L to 1.04 kg/L. Subsequently, top of the gradient was covered by 10 mL citrate anticoagulated whole blood. At the end, the 50 mL test tube was centrifuged at 2767g for 1 ½ hours. In turn, to generate 17 different density fractions by the help of gravity, the underside of the test tube was pierced by a hot needle and the content was allowed to pour out (Milovanovic et al). Throughout this process, each fraction contained about 2 mL. Platelet counts were analyzed using a CELL-DYN 4000 (Abbott Diagnostics, Illinois, U.S.A.) in all fractions. From there, all density fractions were lysed with Triton X-100 (final concentration of 1%) (Sigma-Aldrich, Missouri, U.S.A.). By centrifugation at 2000g for 10 minutes cell fragments were removed. At last, for determination of 5-HT content an ELISA kit (R&D, UK) was put in use.
For the statistical assessment, Microsoft Excel®, ver. 12.0.6514.5000 was utilized. The unpaired Student’s t test were applied for calculating quantitative data. p-values ≤ 0.05 were used to show significance.
The spreading of platelets in the 17 density fractions is shown in Figure 1. Figure 2 presents serotonin content in all density fractions. The latter figure concludes that fractions from FMS (nos. 7-13) have significant more serotonin (p < 0.05) compared to CON. Common for the study groups, platelets with normal and lower density contained more serotonin compared to platelets with higher density.
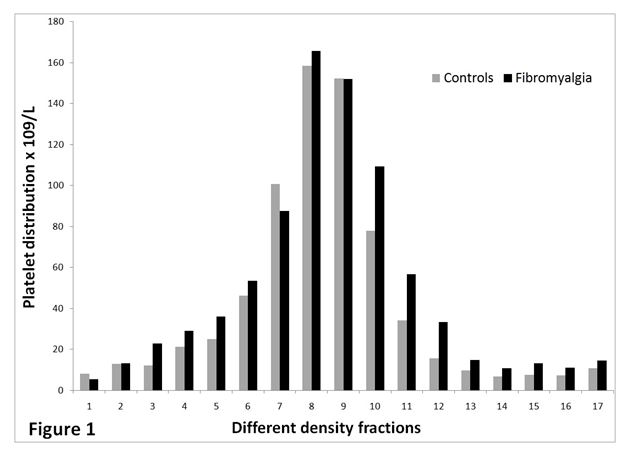
Figure 1. The distribution of platelets (x109/L) in the gradient for AD patients (n=20) and controls (n=16). Fraction no.1 holds platelets having highest density and fraction no. 17 contains platelets with lowest density.
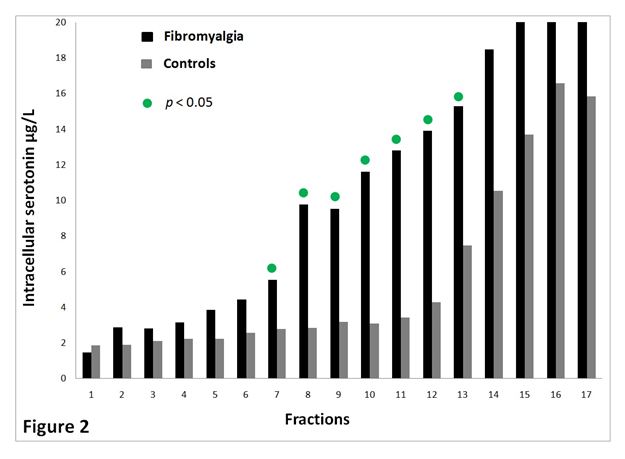
Figure 2. Serotonin (µg/L) content of the different density fractions for FMS (n=20) and CON (n=16). The most dense platelets are found in fraction no. 1. The least dense platelets are located in fraction no.17.
To the best of our knowledge, this is the first report studying FMS with respect to serotonin content in different platelet density subpopulations. It is evident from current study that platelets with normal and lower density from FMS contain augmented levels of serotonin in contrast to same density platelets population from CON group. Upon activation platelets release serotonin, it is therefore to theorize that augmented platelet serotonin content mirrors less in vivo platelet activation. Thus, our current results is indicate opposite, since our recent published data shows that platelets of FMS has a higher platelet activity [36]. One can thereby speculate how much serotonin originate from the platelets and how much is derived from the plasma. It is proposed that low serotonin levels in serum have an inverse correlation with clinical events of apparent pain [37]. Same study also offers an explanation as why platelets may contain high levels of 5-HT, in that platelet 3H-imipramine (5-HT reuptake receptor) binding was higher. It is therefore possible that platelets not only contain serotonin and release upon activation, they also have a reuptake of the molecule, presumable via the SETR. It is further to assume, that serotonin has additional storeroom sites next to dense granules. The results may indicate that an abnormal sensitivity to pain may be due to an instability in the 5-HT system in FMS. This could be a curious scientific finding.
The musculoskeletal pain in FMS is frequently medicated with a range of medicine. Many of those are drugs preventing serotonin uptake in the synapses. The medication increases serotonin concentration in the synaptic cleft. It is therefore to postulate that the neurons eventually release a higher amount of serotonin in FMS. Uptake of serotonin occurs in different parts of the body i.e. by the platelets. FMS individuals thus experience pain in many parts of the body, one can speculate if a connection exists. An additional result is that there are to be a decreased efficiency for these drugs in FMS, and patient will still experience pain problems. Unfortunately, one can conclude that the FMS offers a difficult therapeutical challenge. FMS provide many cases which is not curative, just momentary or limited relief is offered. That alone is not unimportant, but it is also important to make an effort to understand the mechanisms of 5-HT and platelets and thereby better recognize FMS. By doing so, it could provide a help to FMS patients in the future. It may be significant to analyze platelet serotonin levels and also serum levels. However, the clinical impact of platelet serotonin in platelets needs further research.
Circulating 5-HT depends mostly on its production in the gastrointestinal tract [38]. A determinant of human 5-HT synthesis is the accessibility of the precursor tryptophan. It depends on the rate-limiting enzyme tryptophan hydroxylase and its cofactors, tetra-hydrobiopterin and folic acid as well [39]. Therefore, with a high degree of certainty, platelet serotonin content depends on food ingestion. For that reason, it is even possible that nutrition could affect the pain level of the FMS.
- Wolfe F, Smythe HA, Yunus MD, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum 33:160-172. [crossref]
- Wolfe F, Ross K, Anderson J, Russell IJ, Herbert L. (1995) The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 38:19-28. [crossref]
- Hudson JI, Goldenberg DL, Pope HG Jr, Keck PE Jr, Schlesinger L (1992) Comorbidity of fibromyalgia with medical and psychiatric disorders. Am J Med 92: 363-367. [crossref]
- Yunus MB, Dailey JW, Aldag JC, Masi AT, Jobe PC (1992) Plasma tryptophan and other amino acids in primary fibromyalgia: a controlled study. J Rheumatol 19: 90-94. [crossref]
- Neeck G (2002) Pathogenic mechanisms of fibromyalgia. Ageing Res Rev 1: 243-255. [crossref]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, et al. (2001) Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther 92: 179-212. [crossref]
2021 Copyright OAT. All rights reserv
- Oldendorf WH (1971) Uptake of radiolabeled essential amino acids by brain following arterial injection. Proc Soc Exp Biol Med 136: 385-386. [crossref]
- Yuwiler A, Oldendorf WH, Geller E, Braun L (1977) Effect of albumin binding and amino acid competition on tryptophan uptake into brain. J Neurochem 28: 1015-1023. [crossref]
- Mattson MP, Maudsley S, Martin B (2004) BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27: 589-594. [crossref]
- Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60: 355-366. [crossref]
- McNicol A, Israels SJ (1999) Platelet dense granules: structure, function and implications for haemostasis. Thromb Res 95: 1-18. [crossref]
- Paasonen MK (1968) Platelet 5-hydroxytryptamine as a model in pharmacology. Ann Med Exp Biol Fenn 46: 416-422. [crossref]
- Sneddon JM (1973) Blood platelets as a model for monoamine-containing neurones. Prog Neurobiol 1: 151-198. [crossref]
- Lingjaerde O(1969) Uptake of serotonin in blood platelets: Dependence on sodium and chloride, and inhibition by choline. FEBS Lett 3: 103-106. [crossref]
- Shaskan EG, Snyder SH (1970) Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther 175: 404-418. [crossref]
- De Clerck F, Xhonneux B, Leysen J, Janssen PA (1984) Evidence for functional 5-HT2 receptor sites on human blood platelets. Biochem Pharmacol 33: 2807-2811. [crossref]
- Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, et al. (2002) Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 415: 175-179. [crossref]
- Stratz T, Mennet P, Benn HP, Müller W (1991) [Blocking of S2 receptors--a new treatment principle in generalized tendomyopathy (fibromyalgia)?]. Z Rheumatol 50: 21-22. [crossref]
- Lesch KP, Wolozin BL, Murphy DL, Reiderer P (1993) Primary structure of the human platelet serotonin uptake site: identity with the brain serotonin transporter. J Neurochem 60: 2319-2322. [crossref]
- Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C (1996) A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum 39: 1852-1859. [crossref]
- Russell IJ (1998) Advances in fibromyalgia: possible role for central neurochemicals. Am J Med Sci 315: 377-384. [crossref]
- Chamberlain KG and Penington DG. (1988) Monoamine oxidase and other mitochondrial enzymes in density subpopulations of human platelets. Thromb Haemost 59:29-33. [crossref]
- Chamberlain KG, Froebel M, Macpherson J, Penington DG (1988) Morphometric analysis of density subpopulations of normal human platelets. Thromb Haemost 60: 44-49. [crossref]
- Mezzano D, Hwang KL, Catalano P, Aster RH. (1981) Evidence that platelet buoyant density, but not size, correlates with platelet age in man. Am J Hematol 11:61-76. [crossref]
- Boneu B, Vigoni F, Boneu A, Caranobe C, Sie P (1982) Further studies on the relationship between platelet buoyant density and platelet age. Am J Hematol 13: 239-246. [crossref]
- Karpatkin S (1969) Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest 48: 1083-1087. [crossref]
- Corash L, Tan H, Gralnick HR, Shafer B. (1977) Heterogeneity of human whole blood platelet subpopulations: I. Relationship between buoyant density, cell volume, and ultrastructure. Blood 49:71-87. [crossref]
- Penington DG, Lee NL, Roxburgh AE, McGready JR (1976) Platelet density and size: the interpretation of heterogeneity. Br J Haematol 34: 365-376. [crossref]
- Caranobe C, Sie P, Boneu B (1982) Serotonin uptake and storage in human platelet density subpopulations. Br J Haematol 52: 253-258. [crossref]
- Martin JF, Shaw T, Heggie J, Penington DG (1983) Measurement of the density of human platelets and its relationship to volume. Br J Haematol 54: 337-352. [crossref]
- Martin JF, Plumb J, Kilbey RS, Kishk YT (1983) Changes in volume and density of platelets in myocardial infarction. Br Med J (Clin Res Ed) 287: 456-459. [crossref]
- Järemo P, Hansson G, Nilsson O. (2000) Elevated inflammatory parameters are associated with lower platelet density in acute myocardial infarctions with ST-elevation. Thromb Res 100:471-478. [crossref]
- Järemo P, Sandberg-Gertzen H (1996) Platelet density and size in inflammatory bowel disease. Thromb Haemost 75: 560-561. [crossref]
- Järemo P (1999) Platelet density in essential thrombocythemia and polycythemia vera. Platelets 10: 61-63. [crossref]
- Milovanovic M, Lotfi K, Lindahl T, Hallert C, Järemo P. (2010) Platelet density distribution in essential thrombocythemia. Pathophysiol Haemost Thromb 37:35-42. [crossref]
- Milovanovic M, Nilsson S, Harakka PI, Post C, Gerlde B. (2016) High in vivo platelet activity in female fibromyalgia patients. Journal of Biomedical Sciences 3:21.
- Russell IJ, Michalek JE, Vipraio GA, et al. (1992) Platelet 3H-imipramine uptake receptor density and serum serotonin levels in patients with fibromyalgia/fibrositis syndrome. J Rheumatol 19:104-109. [crossref]
- Furness JB and Costa M. (1982) Neurons with 5-hydroxytryptamine-like immunoreactivity in the enteric nervous system: their projections in the guinea-pig small intestine. Neuroscience 7:319-341. [crossref]
- Green CB, Cahill GM, Besharse JC (1995) Tryptophan hydroxylase is expressed by photoreceptors in Xenopus laevis retina. Vis Neurosci 12: 663-670. [crossref]


