Abstract
Background
The autologous implantation technique (ACI) was first introduced in studies on cartilage defects in knees. It has later been used for the treatment of osteochondral knee lesions. The patient presented here had a hip osteochondral defect measuring 4 cm in diameter. He was a candidate for hip prosthesis because of serious pain and reduced function. Being young and highly motivated for alternatives to prosthesis he was to our knowledge the first patient treated with ACI to the hip in 1999.
Methods
Autologous chondrocytes obtained from knee cartilage biopsies were grown at a laboratory in Gothenburg. The operation was done at The University Hospital North Norway where removal of damaged cartilage and loose bony fragments was done on an operatively luxated hip. In the cleaned area implantation of chondrocytes under a periosteal covering was done. Gradual increased weight bearing was allowed postoperatively.
Results
Immediately postoperatively the patient reported significant relief of pain, and in the period of more than 15 following years he generally estimated pain to be 70 % reduced from the preoperative value. Frequent MR examinations the first postoperative year showed gradual filling of the defect in bone and cartilage. Contrast MRI at 22 months depicted the surface of the repaired area quite smooth. At the last control more than 15 years following ACI he reported limited pain, walked without a limp; and moderate osteoarthritis seen previously had developed further. Knowing the result, he was happy to have gone through the procedure.
Conclusion
The patient has gained more than fifteen prosthesis-free years because of the ACI operation. Quite normal cartilage adjacent to the large defect was carrying weight, and the repair area was not destroyed, as probably would have been the case in osteoarthritis. A success on the various attempts to produce normal cartilage in vitro, able to carry some weight immediately after implantation, could in the future open up for improved biological treatment of large defects, both chondral and osteochondral, in hip and knee.
Keywords
cartilage; hip osteochondral defect; chondrocyte implantation; tissue engineering in cartilage
Background
A technique for fixation of loose osteochondral fragments of the hip with nails from transplants of cortical bone was described in 1980. Two patients had been followed for many years after a successful treatment with such nails [1]. A 33 year old man had previously been operated for a hip osteochondral defect with that technique at another hospital, but the lesion failed to heal. He was under consideration for THR because of serious pain and reduced function. The young age of the patient, and his own motivation to find alternatives to THR, influenced our search for a biological repair.
The ACI technique was first used to treat cartilage defects in knees [2]. It inspired new basic and clinical studies, including prospective, randomized studies [3-5]. Osteochondritis dissecans in knees has been treated with ACI with improvements in pain and function also after failed alternative approaches [6,7]. We searched for alternatives to insertion of prosthesis with some technique able to induce repair of the defect in the femoral head. Even though ACI to hip according to our knowledge had not been performed previously, the patient was operated with ACI in 1999 with the motivation to improve hip anatomy and function, reduce pain and delay the need for THR.
Case presentation
A patient born in 1966 had pain and significantly reduced function in his right hip because of advanced osteochondritis dissecans diagnosed in 1984. He was operated in 1987 at another hospital through an anterolateral approach keeping the femoral head in situ. Four 25 mm pieces of cortical bone taken from the tibia had been used as nails in an attempt to fix the loose fragment. In a short period after the operation he had relief of pain, but control radiographs never showed healing of the affected area. Pain and reduced function persisted after the short initial relief. When examined by us in 1998 he had pain starting in the morning, increasing during physical activity, remaining after such activity and being troublesome at night influencing his sleep to a high degree. The well established and reasonable treatment THR was first considered. The young age of the patient, his own motivation for alternatives, and our previous experience using ACI in the knee motivated to use ACI in the hip.
Investigations
Initial radiographs showed a severe osteochondritis dissecans in the right femoral head (Figure 1), with additional early osteoarthritis in the joint. MRI confirmed these findings, and did not show any extensive necrosis of the femoral head.
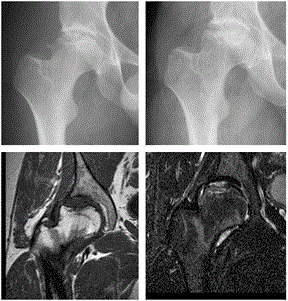
Figure 1. Preoperative and first post-operative x-ray at 8 months. 16 months postoperative coronal 0.5 Tesla MR T1 and STIR, on the STIR image there is only moderate edema around the new cartilage and a slightly irregular femoral articular surface. The articular fluid is brighter than the implanted cartilage.
Treatment
ACI to the right hip was performed in June 1999 after in vitro chondrocyte cultivation from harvested cartilage biopsies from his right knee. At the operation the hip was luxated; all loose cartilage and bony fragments in the osteochondral bed were removed which left a cleaned area with a bony surface measuring four centimetres in diameter. Bleeding from the bone surface was stopped; a periosteal membrane harvested from the patient’s proximal tibia was sutured on, and in vitro grown cells were injected under the periosteal flap. A cautious relocation of the femoral head was done with high awareness of the vulnerability of the repair area. Weight bearing and physical therapy followed after six weeks on crutches, and the patient continued to use one crutch for six months. In one period after twelve years he experienced increased pain in the hip. He improved after advice to do self-administered stretching exercises for what was additionally diagnosed a retrotrochanteric pain syndrome [8-10].
Outcome and follow-up
The patient reported a 70 % pain reduction early after the operation. He reported no pain at night at follow up at 4 months, and has insisted never to be awakened by pain after the ACI operation. At later visits in the outpatient clinic he repeatedly reported a 70 % pain reduction from preoperative values. After he was diagnosed with a retro-trochanteric pain syndrome, he has reported a positive effect of self-administered stretching exercise, which he has used semi-regularly [8]. Repeated MRI examinations at three, six and nine months postoperatively (not shown here), and others at later stages (Figure 1 and Figure 2) showed that a partial filling of the defect in lower part of the repair area developed into a tissue completely filling the defect where the tissue had a smooth border facing the joint. The smooth surface of the head of the femur was especially displayed on contrast MRI (Figure 2).
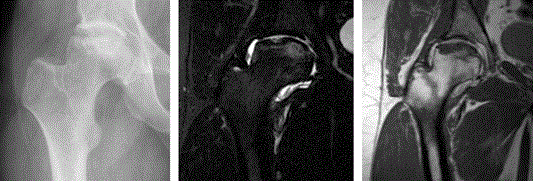
Figure 2. 22 Months follow up x-ray and 1.5 Tesla MR arthrografy. The x-ray remained unchanged; the MR shows still some edema around the implanted cartilage, the small surface irregularity is less conspicuous than before and the articular surface is well depicted due to the intra-articular fluid on both sequences.
At his last outpatient visit in January 2015 he walked without a limp, used no crutches, and was working full time. He reported that in some positions when carrying weight on his operated hip and moving he could feel episodes of pain. The osteoarthritis which had been seen on radiographs before the ACI procedure was more advanced (Figure 3). The area with repair tissue facing the joint space seemed to remain as one entity, but some small cysts were seen some millimetres from the surface of the joint. Summing up his view in retrospect the patient never regretted to have been operated with ACI. He has had no problem with his right knee where cartilage was harvested for the cultivation of cells. Even at his last visit he was not interested in considering THR.
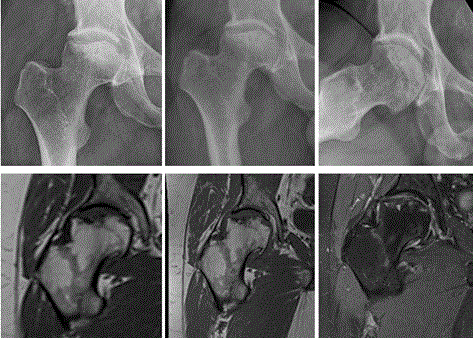
Figure 3: 7(a) and 13(b+c) years follow-up x-rays. 10 years follow up1.5 Tesla MR T1 (d) and 16 years 3Tesla MR follow-up T1(e) and fat suppressed PD(f). The development of hip arthrosis is seen on the x-rays and MR images. There is still only little edema around the implanted cartilage and the articular surface is still smooth, although also less convex than on the first images. Small areas of high signal intensity presumably, subchondral cysts, can be seen on the last MR(f).
Discussion
So far the patient has gained more than fifteen prosthesis-free years using a modified ACI technique for OCD in the hip. Despite some osteoarthritic signs on radiographs, he did not have alarming clinical and radiographic deterioration fifteen years after the operation in 1999. To our knowledge this is the first ACI to a hip. Another patient operated after January 2002 who had sequel after a traumatic damage to the femoral head was treated with an ACI procedure to the hip. In that case manufactured collagen membrane was used instead of periosteum. ACI was argued to be a possible method for the treatment of massive cartilage loss in young adults in some cases [11].
Our patient had a large defect measuring 4 cm in diameter. It was larger and deeper than the maximum of 10 cm2 included in the prospective, randomized Norwegian study comparing ACI and microfracture in the knee [3]. We have proof that stem cells are located in the subchondral region, and bleeding may release them into an area to be repaired [12]. Classical microfracture treatment would probably have exposed the large repair area to a high degree of mechanical irritation and washout of cells, so inducing bleeding from bone obviously must have been combined with covering with a membrane [13]. The potential influence of inducing bleeding from underneath bone in an area where ACI is done remains to be evaluated. In this case we meticulously stopped bleeding before suturing the periosteal patch. The damaged area was filled gradually by repair tissue especially during the first nine months. It actually took a year until MRI showed a nice and smooth surface of the femoral head. This gradual production of tissue seen on repeated MRI illustrates a need for protection of the new tissue developing during repair. The sharp border between abnormal and normal cartilage created shoulders of macroscopically quite normal tissue surrounding the defect after debridement, which is difficult to achieve in cases of osteoarthritis. These shoulders could carry weight postoperatively and reduce mechanical irritation on the repair area
The impression of a quite well repaired area seen after two years on MRI has remained quite unchanged. The latest MRI has however showed more developed osteoarthritic changes and some cysts under the repair tissue.
Worldwide research is ongoing to find methods for tissue engineering of cartilage in vitro [13-17]. In vitro construction of sheets of cartilage for implantation into damaged joints might in the future be a solution for faster normalization than with the use of cells. Stem cells mixed in bleeding from bone may help attach such sheets of in vitro produced cartilage both to bone and surrounding cartilage after implantation, and both osteochondral as well as osteoarthritic pathology could more efficiently be repaired. The method used in the present case probably will fit only where remaining cartilage surrounding a limited defect can carry weight postoperatively. Probably some product of an in vitro produced sheet of cartilage and a good method for fixation in the joint can widen the indication for cartilage repair also in hip.
Acknowledgements
2021 Copyright OAT. All rights reserv
Our thanks to Dr. Bjarne Andreas Olsen who suggested ACI for the patient, Dr. Johan Fredrik Winge who contributed significantly during the operation, and Director Knut Schrøder who facilitated translational research at the hospital.
References
- Lindholm TS, Osterman K (1980) Internal fixation of the fragment of osteochondritis dissecans in the hip using bone transplants. A report of two cases. J Bone Joint Surg Br 62-62B: 43-5. [crossref]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, et al. (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331: 889-895. [crossref]
- Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, et al. (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86-86A: 455-64. [crossref]
- Van Assche D, Staes F, Van Caspel D, Johan V, Johan B, et al. (2010) Autologous chondrocyte implantation versus microfracture for knee cartilage injury: a prospective randomized trial, with 2-year follow up. Knee Surg Sports Traumatol Arthrosc 18: 486-495.
- Bentley G, Biant LC, Vijayan S, Macmull S, Skinner JA, et al. (2012) Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J Bone Joint Surg Br 94: 504-509. [crossref]
- Cole BJ, DeBerardino T, Brewster R, Farr J, Levine DW, et al. (2012) Outcomes of autologous chondrocyte implantation in study of the treatment of articular repair (STAR) patients with osteochondritis dissecans. Am J Sports Med 40: 2015-2022. [crossref]
- Peterson L, Minas T, Brittberg M, Lindahl A (2003) Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 2: 17-24. [crossref]
- Meknas K, Kartus J, Letto JI, Flaten M, Johansen O (2009) A 5-year prospective study of non-surgical treatment of retro-trochanteric pain. Knee Surg Sports Traumatol Arthrosc 17: 996-1002. [crossref]
- Meknas K, Johansen O, Kartus J (2011) Retro-trochanteric sciatica-like pain: current concept. Knee Surg Sports Traumatol Arthrosc 19: 1971-1985. [crossref]
- Meknas K, Christensen A, Johansen O (2003) The internal obturator muscle may cause sciatic pain. Pain 104: 375-380. [crossref]
- Akimau P, Bhosale A, Harrison PE, Roberts S, Mccall IW, et al. (2006) Autologous chondrocyte implantation with bone grafting for osteochondral defect due to posttraumatic osteonecrosis of the hip- a case report. Acta Orthopaedica 77: 333-336. [crossref]
- Elvenes J, Knutsen G, Johansen O, Moe BT, Martinez I (2009) Development of a new method to harvest chondroprogenitor cells from underneath cartilage defects in the knees. J Orthop Sci 14: 410-417. [crossref]
- Fontana A, de Girolamo L (2015) Sustained five-year benefit of autologous matrix-induced chondrogenesis for femoral acetabular impingement-induced chondral lesions compared with microfracture treatment. Bone Joint J 97-97B: 628-35. [crossref]
- Bhattacharjee M, Coburn J, Centola M, Murab S, Barbero A, et al. (2015) Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev 84:107-22. [crossref]
- Yousefi AM, Hoque ME, Prasad RG, Uth N (2015) Current strategies in multiphasic scaffold design for osteochondral tissue engineering: A review. J Biomed Mater Res A 103: 2460-2481. [crossref]
- Athanasiou KA, Eswaramoorthy R, Hadidi P, Hu JC (2013) Self-organization and the self-assembling process in tissue engineering. Annu Rev Biomed Eng 15: 115-136. [crossref]
- DuRaine GD, Brown WE, Hu JC, Athanasiou KA (2015) Emergence of scaffold-free approaches for tissue engineering musculoskeletal cartilages. Ann Biomed Eng 43: 543-554. [crossref]



