Background: Bimatoprost is a prostamid analogon. It has been successfully used for many years in the treatment of glaucoma. Increased lash growth has been detected as an adverse reaction in using this drug. This implicates that treatment of the skin at the upper and lower lashlines with bimatoprost 0.3 mg/ml ophthalmic solution may lead to eyelash-length enhancement, resulting in a better appearance in aesthetic terms.
Material and methods: To prove its effectiveness on lash growth bimatoprost 0.3 mg/ml ophthalmic solution has been applied twice a day for a six-week period on the outer rim of the right upper eyelid in 15 volunteers. The untreated left eyelid was used as control. Lash growth was documented by clinical photography before and after the treatment period. Furthermore, there was a standardized patient-questionnaire to be answered.
Results: Within a six-week period of topical use of bimatoprost obvious enhancement in lenght of the eyelashes occurred. Photo-documentation showed the difference in lash lengths on the right and left upper eyelids in 9 of 12 participants (75%). In a standardized questionnaire almost 40% of participants reported short-term, reversible side effects like reddening of the eye, foreign body sensations, itching and enhanced growth of hair off the lashline. A statement regarding thickness and darkness of the lashes could not be given, 85% would recommend the product to others.
Discussion: Our results support the concept of using bimatoprost 0.3 mg/ml ophthalmic solution as an eyelash enhancing interval therapy for the treatment of eye lash hypotrichosis or for aesthetic purposes. Despite the clear positive effects on lash growth, there is still a certain risk of unwanted side effects. Users have to take this under consideration. Further controlled studies have to enlighten the effect of bimatoprost on the abbreviation of the telogen phase of the eyelashes.
bimatoprost, eyelashes, lash growth, hypotrichosis, prostamid, anagen, telogen
Long eyelashes have always been a symbol of beauty and attractiveness. There are many ways to increase lash lenght, thickness and darkness like using different types of mascara, false lashes, lash transplantation and a variety of sera.
The hair cycle of eyelashes lasts five to twelve months: anagen-phase one to two month, catagen-phase 15 days and telogen-phase four to nine months [1]. The lenght of the hair depends on the duration of anagen-phase [2,3]. Bimatoprost, a prostamid analogon (C25H37NO4), has been used successfully for decreasing the intraocular pressure in chronic open-angle glaucoma and ocular hypertension for decades. Increased lash growth has been observed as an adverse drug reaction. This effect is supposedly related to a prolongation of the anagen phase, the growth phase of the hair cycle. According to the literature it may also lead to an increase of thickness and darkness of the eyelashes [1,4-8]. Therefore Latisse® (bimatoprost ophthalmic solution 0.03%) was FDA approved for the treatment of hypotrichosis of the eyelashes in the United States in December 2008 [4].
As this product is not available in Europe, we designed an off-label-use study utilizing bimatoprost 0.3 mg/ml eye drops (Lumigan®) for the enhancement of lash growth.
Recruiting: Healthy volunteers have been recruited by announcement in the polyclinic of the Department of Dermatology at the Medical University of Graz. Fourteen female and one male test persons (age 20 to 34 years, mean 26) were included in consideration of the inclusion and exclusion criteria (Table 1). According to GCP-regulations they could reject their participation at any time. Before study start informed consent has been signed by each participant. The study was approved by the ethics committee of the Medical University of Graz.
Table 1: Inclusion and exclusion criteria
Inclusion criteria |
- Persons of both sexes between 18 and 99 years
- Signed informed consent before the start of the study
- Effective contraception in women of child-bearing potential [as per description of the ethics commission Austria
|
Exclusion criteria
|
- Use of contact lenses during the study
- Use of eye drops (indication and product irrelevant) during the last six month
- Pregnant women or women of child-bearing potential without an effective contraception
- Known hypersensitivity or allergy to Bimatoprost or one of the other ingredients of Lumigan® 0,3 mg/ml ophtalmic solution
|
Study design: Bimatoprost 0.3 mg/ml ophthalmic solution was tested for its effectiveness on lash growth on the upper eyelid of the right eye, the left upper eyelid served as a control (half-side-trail). Lash length was documented by clinical photography before and after the treatment period. Both eyes were photographed without and with black mascara, as well as without and with scale. For duration of six weeks all participants had to apply the study drug two times every day. At the end they had to answer a short questionnaire concerning side effects and patient satisfaction.
For the duration of the study no eyeliner or any sort of eye drops should be used.
Fifteen healthy volunteers were included, two dropped out because of a non-itching reddening of the eye, one after two, the second after four days. One person confessed irregular use, her questionnaire was evaluated, and her pictures were not.
Therefore we analysed the clinical photography of 12 participants and the questionnaires of 13.
Images
The overview pictures (both eyes) were taken for the analysis with the naked eye.
No statement concerning a change in thickness or darkness could be given.
Scoring was performed by comparing the length enhancement of the lashes on the right and left eyelid: 0 meaning no change and + 1 meaning visible longer lashes on the treatment side (right upper eyelid). Images were evaluated by three independent physicians from the Department of Dermatology. There was a 100%-match of the results (Table 2).
Table 2: Analysis of the Images
Rating |
Number of Participants – Baseline |
Number of Participants – End Date* |
+ 1 |
0 |
9 |
0 |
12 |
3 |
* Six weeks after the start of the study |
As expected at the beginning of study there was no difference in the length of the lashes. After the six-week period of topical use of bimatoprost on the right upper eyelid there was an obvious difference in length of the eyelashes in 75% of the participants, 25% showed no assessable difference. Examples of rated images can be seen in Table 3.
Table 3: Examples of rated Images (End Date)
ID |
Image |
Score |
OS94 |
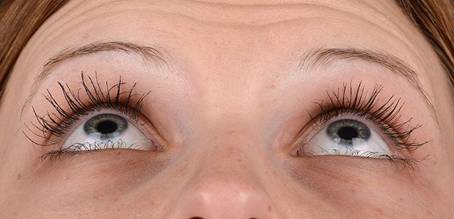
|
+ 1 |
MD90 |
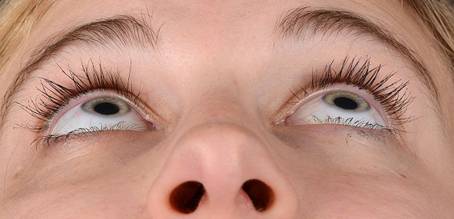
|
+ 1 |
LS87 |
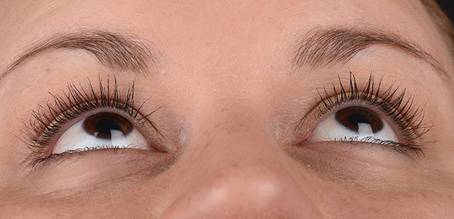
|
+ 1 |
BS80 |
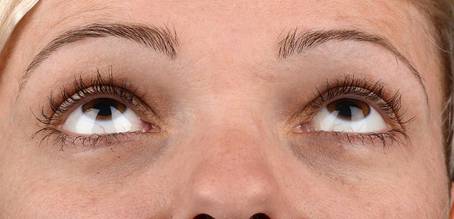
|
+ 1 |
Standardized patient questionnaire
11 of 13 participants (84.7%) were satisfied or very satisfied with the product. All test persons were satisfied or very satisfied with the mode of application of the product. 9 of 13 participants (69.2%) claimed that there was an increased lash growth. 84.7% of the participants would recommend the product to others.
38.5% of them reported short-term, reversible but unwanted side effects like reddening of the eye, foreign body sensations, itching and enhanced growth of hair underneath the lashline. These effects were part of the expected side effect profile (Table 4).
Table 4: Questionnaire evaluation
Question |
Answer |
Absolute frequency |
Relative frequency |
How satisfied were you with the product? |
Very satisfied |
5 |
38.5% |
Satisfied |
6 |
46.2% |
Not very satisfied |
2 |
15.3% |
Not satisfied |
0 |
0% |
How satisfied were you with the application? |
Very satisfied |
8 |
61.5% |
Satisfied |
5 |
38.5% |
Not very satisfied |
0 |
0% |
Not satisfied |
0 |
0% |
Did you recognise an obvious lash growth on the right eye during the last six weeks? |
Yes |
3 |
23.1% |
Rather yes |
6 |
46.2% |
Rather no |
4 |
30.7% |
No |
0 |
0% |
Have you recognised any side effects during the last six weeks? |
Yes |
5 |
38.5% |
No |
8 |
61.5% |
If you could buy the product*, how much money would you spend? |
< 50 € |
6 |
46.2% |
50 € |
6 |
46.2% |
75 € |
1 |
7.6% |
100 € |
0 |
0% |
> 100 € |
0 |
0% |
Would you recommend the product to others? |
Yes |
11 |
84.7% |
No |
2 |
15.3% |
* Contains the amount of Bimatoprost 0.3 mg/ml ophthalmic solution for a six-week period of topical use on both eyes |
A statement regarding thickness and darkness of the lashes could not be given.
Bimatoprost, used for the treatment of chronic open-angle glaucoma and ocular hypertension, has shown undesired eye-lash growth as a side effect [1,4,5,7,9-13]. Harii et al. (2014) demonstrated increased eyelash growth for aesthetic purposes in 29.5% of the participants after one month, 48.9% after two, 77.3% after three and 78.6% after four month [9].
Our study underlines that topical use of bimatoprost on the eyelids leads to an obvious enhancement in lenght of eyelashes in 75% of the participants after a six-week period. In our study almost 40% of the participants showed side effects. This number is similar to comparable studies from Harii et al. and Smith et al. [10,12].
As the anagen-phase lasts one to two months [6] bimatoprost leads to a prolongation of this phase of the hair cycle [4,5,12]. Randall et al. stated that the telogen-phase of eyelids lasts four to nine months [3]. So far, bimatoprost-induced changes of the duration of the telogen phase have not been explored yet. Our findings suggest that the prolongation of the anagen phase could perhaps cause a shortening of the telogen phase of the eyelash growth cycle. Thus, further studies have to be conducted to newly define the duration of the growth cycle phases of eyelashes under topical treatment with prostamid analoga.
Dana Moore, M. D., Erika Richtig, M. D., and Daisy Kopera, M. D. had full access to all of the data in this work and take responsibility for the integrity of the data and accuracy of the data analysis.
- Fagien S (2010) Management of hypotrichosis of the eyelashes: Focus on bimatoprost. Clin Cosmet Investig Dermatol 3: 39–48. [Crossref]
- Sobotta J, Welsch U, Sobotta-Welsch (2009) Lehrbuch Histologie: Zytologie, Histologie, mikroskopische Anatomie (2ndedtn), Elsevier Urban & Fischer, München.
- Randall VA (2007) Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol 18: 274–285. [Crossref]
- Cohen JL (2010) Enhancing the growth of natural eyelashes: the mechanism of bimatoprost-induced eyelash growth. Dermatol Surg 36: 1361–1371. [Crossref]
- Wester ST, Lee WW, Shi W (2010) Eyelash growth from application of bimatoprost in gel suspension to the base of the eyelashes. Ophthalmology 117: 1024–1031. [Crossref]
- Johnstone MA, Albert DM (2002) Prostaglandin-induced hair growth. Surv Ophthalmol 47: 185–202. [Crossref]
- Yoelin S, Walt JG, and Earl M (2010) Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg 36: 638–649. [Crossref]
- Elias MJ, Weiss J, and Weiss E (2011) Bimatoprost ophthalmic solution 0.03% for eyebrow growth. Dermatol Surg 37: 1057–1059.
- Tauchi M, Fuchs TA, Kellenberger AJ, Woodward DF, Paus R, et al. (2010) Characterization of an in vivo model for the study of eyelash biology and trichomegaly: mouse eyelash morphology, development, growth cycle, and anagen prolongation by bimatoprost. Br J Dermatol 162: 1186–1197. [Crossref]
- Smith S1, Fagien S, Whitcup SM, Ledon F, Somogyi C, et al. (2012) Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J Am Acad Dermatol 66: 801–806. [Crossref]
- Khidhir KG1, Woodward DF, Farjo NP, Farjo BK, Tang ES, et al. (2013) The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J 27: 557–567. [Crossref]
- Harii K, Arase S, Tsuboi R, Weng E, Daniels S (2014) Bimatoprost for eyelash growth in Japanese subjects: two multicenter controlled studies. Aesthetic Plast Surg 38: 451–460. [Crossref]
- Ahluwalia GS (2013) Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc 16: S73-76. [Crossref]




