Objective: Explain the observation of the the mechanism of an “overgrown brain” in a C57bl6 mouse model after exposure of juvenile individuals for 40 days to a High Fat diet based on bovine lard.
Methods: 8-12 weeks old C57Bl6 mice were fed for 40 days a high-fat diet (+025% cholesterol 45% energy from bovine lard). With LC-MS techniques we measured Cholesteryl esters(ChE), lysophosphatidyl-cholines (LPC), phosphatidylcholine (PC), sphingomyelin (SPM) and Triacylglycerol’s (TG) in brain tissue after 40 days of exposure to a High Fat diet or nutritional intervention of two days starvation.
Results: Major observation was that mainly unsaturated Very Long Chain Fatty Acids (TGs) of the C:50, C:52, C:54 and C56 TGs fraction were extremely accumulated in this HF-diet and were strongly correlated with the TGs with the HF-diet obese C57BL6 whole brain fraction (correlation coefficient r2=07.60 in comparison to control chow r2= 0.264). In addition, “brain steatosis” in the HF-diet group was also caused by a non-significant 135% increase of Docosahexaenoic Acid ([DHA] C22:6Ω3) in the HF-diet group in the brain. LPC and SPM were strongly significantly decreased in the starvation group (P<0.0001), followed by a strong decrease of PC and PE (P<0.007), followed by the DG fraction (P<0.017). In addition, ChE strongly significantly increased in the HF-diet group (P<0.001).
Conclusions: From our observations we can conclude a juvenile rodent study showed in a by bovine lard induced obesity model an “overgrown brain” mainly by the accumulation of triacylglycerols (TGs). These observations of “brain steatosis” in a C57bl6 mouse model after a High Fat diet based on bovine lard in combination with the literature data of the anatomically found strict correlation between maternal Body Mass Index (BMI) and head circumference of new-born elevates our observations to the evolutionary research area of human encephalization.
brain steatosis, obese mouse model, high fat diet, starvation, LCMS, Lipids, Systems Biology, Lipidomics, fat, triacylglycerols, Docosahexaenoic Acid (DHA), brain composition, human encephalization, anthropromorphic index, head circumference newborn, encephalization
The response of an organ to a condition of starvation or a High-Fat [HF] diet is tissue dependent and most tissues can use a variety of substrates for energy metabolism, ie, they are capable of utilizing lipids and proteins as well as carbohydrates. Until recently it was hypothesized that the brain’s incapacity to utilize energy sources other than glucose and ketone bodies [1], constitutes a mechanism of protecting it from self- degradation, at the expense of the rest of the body during starvation [2,3]. This projection is based upon the brain’s believed inability to catabolize substances of which it is built, for energy production. In addition, Fatty Acids (FA) do not serve as fuel for the brain, because they are bound to plasma albumin and so do not traverse the blood-brain barrier (BBB) [1].
So, until recently the brain was considered as an exception, which was mostly exclusively dependent on glucose and ketone bodies [1]. Under normal nutritional conditions systemic glucose is the only significant source of energy for the brain, while under conditions of starvation, body carbohydrate reservoir diminishes first, blood glucose level drops and lipid catabolism is enhanced [3]. The liver produces ketone bodies as by-products of excess lipid catabolism which is under these conditions an important “fuel” for the brain in combination of a minimal amount of glucose levels produced by gluconeogenesis [1,3]. Lipids cover about 60% of the brain’s dry weight making brain tissue the second most lipid-dense tissue after adipose tissue [4]. It is suggested that the brain’s inability to use fatty acids and amino acids for energy, constitutes a mechanism of protecting it from self-destruction in order to maintain its integrity, at the expense of the rest of the body during starvation. This protection is based upon the brain’s inability to catabolize substances of which it is built, for energy production [1]. In order to investigate this topic, we compared two different diet interventions that caused hyperlipidemia ie fasting for 2 days and a high-fat diet (45 energy % from bovine lard + 025% cholesterol) for 40 days in whole brains of a C57/BL6 mouse model. This study was designed in order to study type 2 diabetes in several organs and tissues of an obese high fat diet induced C57bl6 mouse model [5]. Our initial hypothesis was that the brain in an obese C57bl6 mous model was unaffected by this high fat diet intervention including under conditions of starvation.
Experimental protocol
In order to explain this topic what the effects were of a high fat diet and 48 hours starvation in a C57bl6 mouse model on brain tissue we used a Systems Biology, lipidomics based approach with LC-MS techniques. Purebred male wild-type C57bl6 mice (age 8-12 weeks, all male), obtained from Charles River (Maastricht, The Netherlands) were used. Three treatments were compared: A). A regular diet (Control, n=6); B). A high-dietary situation in which mice received a High-Fat diet during 40 days and then were sacrificed (HF, n=7) and C). 48 hours starvation (STA, n=8). Mice were housed in a temperature-controlled room (21°C) on a 10-hour dark/14-hour light cycle in group cages. Animal experiments were approved by the animal experimentation committee of the Leiden University Medical Centre (The Netherlands).
Diet
Mice in the Control group were fed a standard lab chow (SDS3, Special Diet Services, Witham, UK) contained about 43 energy percent fat (Table 1). The High-fat diet that was fed to mice in the Treatment group contained 21.4 % protein, 36% carbohydrates, 24% fat, 6% fibers and 57% water (weight-percentages). Before the experiment started animals of both the control group and the treatment group received unrestricted amounts of food and water. Starvation mouse (STV) were deprived from feed for 48 hours. Mice in the Control group fasted for 4 hours before the start of the experiment in order to standardize their metabolic rate.
Table 1. Food composition of the mice chow: “normal” for Control group (Special Diet Services, SDS No3, Witham, UK) and the High-Fat≈ “fatty diet” (Arie Blok, food code 403205, Woerden, The Netherlands) based on bovine lard and 025% cholesterol.
Proximate Analysis |
Standard (SDS3) |
Proximate Analysis |
Fatty diet (403205) |
Moisture (%) |
10.00 |
Moisture (%) |
5.74 |
Crude Oil (%) |
4.25 |
Crude Fat
(Bovine Lard) (%) |
24.00 |
Crude Protein (%) |
22.39 |
Crude Protein (%) |
21.44 |
Crude Fiber (%) |
4.21 |
Crude Fiber (%) |
6.16 |
Ash |
7.56 |
Minerals |
2.25 |
Nitrogen Free Extract |
51.20 |
Nitrogen Free Extract |
36.19 |
----- |
- |
Cholesteryl |
0.25 |
TOTAL |
99.61 |
TOTAL |
96.03 |
Energy (measured bomb-calorimetry) [kJ/g dm] |
16.86 |
Energy (measured bomb-calorimetry) [kJ/g dm] |
21.46 |
LC-MS of lipids and fatty acids
Twenty-one mice were randomly assigned to one of three treatments. Treatment A was the control group (CO) (n=6) and received ad-lib standard lab chow and water during 40 days. Treatment B were animals that received a High-Fat Diet during 40 days (HF) (n=7) while Treatment C consisted of a starvation group (STA) (n=8) which was exposed to food deprivation for a period of 48 hours.
Brain tissue
A brain homogenate (~10% wet weight/ vol) in PBS (phosphate-buffered saline) was made by stirring the tissue in a closed tube with small glass beads.
Mass spectrometry (LC-MS)
As described earlier [6-12] fifty μl of the well mixed tissue homogenate was mixed with 1000 μl IPA containing 4 internal standards. In addition blood plasma samples of 10 µl plasma were extracted with 300 µl of isopropanol (IPA) containing several internal standards (IS: C17:0 lysophosphatidylcholine, di-C12:0 phosphatidylcholine, tri-C17:0 glycerol ester, C17:0 cholesteryl ester and heptadecanoic acid (C17:0)). Samples were placed in an ultrasonic bath for 5 minutes. After mixing and centrifugation (10000 rpm for 3 minutes) the supernatant was transferred to an autosampler vial. Thereaftere10 μl of the sample was injected on the LC-MS Instrument (Thermo Electron, San Jose, USA). A Thermo LTQ is a linear ion-trap LC-MS instrument (Thermo Electron, San Jose, USA). Lipids were separated on a 150 x 32 mm id C4 Prosphere column (Alltech, USA) using a methanol gradient in 5 mM ammonium acetate and 0.1% formic acid (mobile phase A: 5% methanol, mobile phase B: 90% methanol). The flowrate was 0.4 ml/min and the gradient was as follows: 0-2 min – 20%B, 2-3 min – 20% to 80%B, 3-15 min – 80% to 100%B, 15-25 min – hold 100%B, 25-32 min –condition at 20% B. The instrument used was a Thermo LTQ equipped with a Thermo Surveyor HPLC pump Data were acquired by scanning the instrument from m/z 300 to 1200 at a scan rate of approximately 2 scans/s in positive ion ESI mode.
Analysis of plasmalogens
Analysis of cholesterylesters (ChE), lysophosphatidyl-cholines (LPC), phosphatidylcholine (PC), sphingomyelin (SPM) and triacylglycerols (TG) were based on molecular mass and retention time using internal standards. Because no standards for the plasmalogens exist, mass lists determined by using atmospheric pressure chemical ionization (APCI) and electrospray ionization (ESI) mass spectrometry (MS) techniques were used [11,13]. These mass lists were published on internet www.byrdwell.com/plasmogens and www.byrdwell.com/-PhosphatidylEthanolamine.
Calculations and statistics
For all parameter, the mean value of the control mice group was compared to the mean value of the fatty-diet group. Statistics were performed via SPSS [14] using a two-tailed T-Test for differences between the Control group and both Treatment groups (High-Fat and Starvation) group. P£ 005 was considered as statistically significant. Normality of the data and homogeneity of variances were checked by Kolmogorov-Smirnov and Fmax tests, respectively.
In Table 2 were the results of the LC-MS measurements given. We measured with this advanced technique with moleculair separation based on mass and polarity the following major lipid fractions: Lysophosphatidyl-cholines (LPC), Diacylglycerols (DG); Phosphatidylcholines (PC), Phosphatidylethanolamines (PE), Spingomyelines (SPM); Cholesterylesters (ChE), Triacylglycerols (TGs) while the Phosphatidylcholine-plasmogen (PCplas) and Phosphatidylethanolamines-plasmogen (PEplas) were indirectly determined. From Table 2 we can observe that LPC and SPM were strongly significantly decreased in the starvation group (P<0.0001), followed by a strong decrease of PC and PE (P<0.007), followed by the DG fraction (P<0.017). In addition, ChE strongly significantly increased in the HF-diet group (P<0.001). TGs increased non-significantly between CO and HF-diet group due to a large standard deviation caused by large individual differences.
Table 2. Major Lipid Fractions measured using LCMS techniques following a Systems Biology lipidomics based approach in the whole mouse brain tissue of a C57bl6 model consisting of the following three groups: Control (CO) C57bl6 mouse strain (n=6), a High-Fat Diet (HF) (n=7) and a 48 hours starvation (STA) (n=8). See also Figure 1 & 2 Statistics were performed using a two-tailed T-Test where *: denotes P<0.05; **: P<0.01; ***:P<0.001.
Lipid
Classes |
CO
mean |
CO
std |
HF
Mean |
HF
std |
STA
Mean |
STA
std |
T-test
CO-HF |
T-test
CO-STA |
T-test
HF-STA |
LPC |
0.940 |
0.246 |
1.027 |
0.110 |
1.394 |
0.172 |
0.451 |
0.0041** |
0.0001*** |
DG |
0.580 |
0.131 |
0.763 |
0.198 |
0.505 |
0.153 |
0.073 |
0.344 |
0.017* |
PC |
34.135 |
5.506 |
36.149 |
1.289 |
32.740 |
2.565 |
0.418 |
0.584 |
0.007** |
PE |
1.178 |
0.163 |
1.296 |
0.083 |
1.392 |
0.201 |
0.153 |
0.048 |
0.007** |
SPM |
0.852 |
0.172 |
1.008 |
0.105 |
0.645 |
0.154 |
0.089 |
0.042* |
0.0001*** |
Pcplas |
4.344 |
0.888 |
4.892 |
0.500 |
5.188 |
0.904 |
0.218 |
0.108 |
0.443 |
Peplas |
3.014 |
0.606 |
3.444 |
0.419 |
3.792 |
0.976 |
0.179 |
0.093 |
0.382 |
ChE |
0.069 |
0.011 |
0.102 |
0.016 |
0.136 |
0.167 |
0.001** |
0.295 |
0.586 |
TG |
0.091 |
0.063 |
1.146 |
2.221 |
0.137 |
0.202 |
0.256 |
0.562 |
0.275 |
Total |
45.203 |
7.173 |
49.828 |
3.213 |
45.929 |
4.453 |
0.190 |
0.833 |
0.072 |
From Figure 1 we can make the observation -based on LCMS-data of whole mouse brain- of “brain steatosis” under two nutritional conditions. The specific molecular structure of bovine lard (high amounts of unsaturated C:50-1; C50-2; C:52-2; C:52-3; C54-3;C:54-4 and C56-3 Triacylcholesterols) are depicted in Figure 2. The results from this experiment are indicative that mainly unsaturated Very Long Chain Fatty Acids (TGs) of the C:50, C:52, C:54 and C56 TGs fraction of this this HF-diet are strongly correlated with the TGs of the HF-diet obese C57BL6 whole brain fraction (correlation coefficient r2=0760 in comparison to control chow r2= 0264; Compare Figure 1 and Figure 2). So from our observations we can conclude for a juvenile rodent study in a by bovine lard induced obesity model it shows an “overgrown brain” mainly by the accumulation of triacylglycerols (TGs) based on high amounts of unsaturated C:50-1; C50-2; C:52-2; C:52-3; C54-3;C:54-4 and C56-3 TGs. In addition “brain steatosis” in the HF-diet group was also caused by a non-significant 135% increase of Docosahexaenoic Acid ([DHA] C22:6Ω3) in the HF-diet group in the brain (Table 3) [15-20].
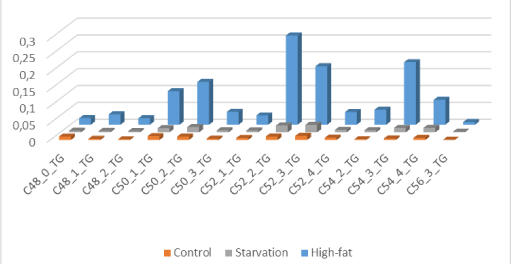
Figure 1. “Brain steatosis” based on LCMS-data of whole mouse brain -“brain steatosis”- under two nutritional conditions: The specific molecular structure of bovine lard (high amounts of unsaturated C:50-1; C50-2; C:52-2; C:52-3; C54-3; C:54-4 and C56-3 Triacylcholesterols.
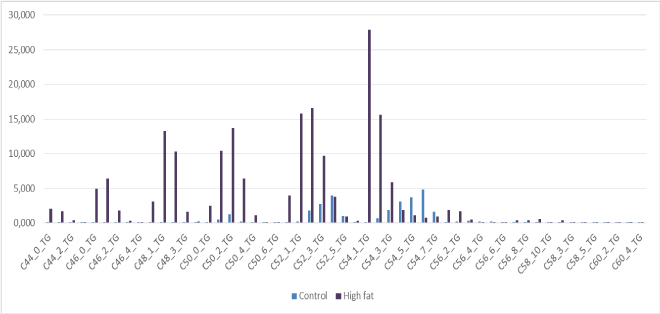
Figure 2. Food composition for the triacylglycerols for Control-Chow mouse diet (425% crude oil without cholesterol; Special Diet Services, SDS No3, Witham, UK) and High-Fat diet based on based on based on 24% Bovine Lard and 0.25% Cholesterol (Arie Blok, food code 403201, Woerden, The Netherlands).
Table 3. Significant changes in Cholesteryl-esters measured with LC-MS techniques in the whole brain of black six mice fed for 40 days a High-Fat Diet containing 0.25% cholesterol (Ch) and 24% energy from bovine lard.
Compound |
Control Diet (A)
(Mean ± SD) |
Starvation
(B)
(Mean ± SD) |
High-Fat Diet (C)
(Mean ± SD) |
Kruskal – Wallis |
P-value |
Change in % |
A vs. B |
A vs. C |
B vs. C |
A vs. B |
A vs. C |
B vs. C |
C16-0-ChE |
0.0028 ± 0.0005 |
0.0069 ± 0.0086 |
0.0049 ± 0.0011 |
*0.022 |
0.2716 |
***0.0009 |
0.5624 |
245.24 |
175.90 |
71.73 |
C16-1-ChE |
0.0060 ± 0.0008 |
0.0130 ± 0.0196 |
0.0102 ± 0.0024 |
**0.005 |
0.4079 |
**0.0021 |
0.7184 |
215.47 |
169.51 |
78.67 |
C18-0-ChE |
0.0149 ± 0.0009 |
0.0160 ± 0.0016 |
0.0161 ± 0.0017 |
0.580 |
0.1571 |
0.1563 |
0.9220 |
107.43 |
108.00 |
100.53 |
C18-1-ChE |
0.0229 ± 0.0017 |
0.0407 ± 0.0443 |
0.0314 ± 0.0046 |
**0.004 |
0.3483 |
**0.0013 |
0.5915 |
178.01 |
137.35 |
77.16 |
C18-2-ChE |
0.0042 ± 0.0016 |
0.0163 ± 0.0314 |
0.0087 ± 0.0029 |
*0.017 |
0.3663 |
**0.0058 |
0.5351 |
392.15 |
209.09 |
53.32 |
C20-3-ChE |
0.0010 ± 0.0004 |
0.0026 ± 0.0035 |
0.0032 ± 0.0010 |
**0.002 |
0.3049 |
***0.0004 |
0.6531 |
255.21 |
318.29 |
124.72 |
C20-4-ChE |
0.0118 ± 0.0043 |
0.0156 ± 0.0078 |
0.0201 ± 0.0037 |
**0.006 |
0.3110 |
**0.0032 |
0.1818 |
131.56 |
169.98 |
129.20 |
C22-6-ChE
(EPA) |
0.0057 ± 0.0026 |
0.0250 ± 0.0508 |
0.0077 ± 0.0013 |
0.154 |
0.3743 |
0.1005 |
0.3847 |
440.97 |
135.14 |
30.65 |
This research manuscript, is the first study where “brain steatosis” is observed in a mouse model. The metabolic effects of randomized fats, has been sparsely studied in humans, and literature data to date is mostly confined to rodent studies [17,18,21].
Both fasting and the high fat diet caused “brain steatosis” (Figure 1, Table 1), mainly due to Triacylglycerols (TGs) accumulation from bovine lard in combination with a non-significant 135% increase of Docosahexaenoic Acid ([DHA] C22:6Ω3) in the HF-diet group towards the brain (Table 2). Like we earlier described for the liver the observation of hepatic steatosis during conditions of “feast” and “famine” [2] –as an evolutionary paradox [2]-, starvation resulted also in this study in a “brain steatosis’’ which can be explained for the starvation group by the essential decline of Phoshophatidylcholine (PC) –the major constituent of the neuron- resulting in significant increase of Lysophosphatidylcholine (LysoPC) a preferred carrier as byproduct of PUFAs across the blood-brain barrier [16] (Tabel 1). In addition, several studies with rodent models are indicative that FA can traverse the Blood Brain Barrier (BBB) [17,18]. In studying High-Fat (HF) diet induced obesity, Insuline Resistance (IR) and type 2 diabetes (T2DM) by lipidomics based LC-MS techniques using a Systems Biology approach, we found unexpectedly a non-significant increase of 1000% up to >10,000% of several Triacylglycerol compounds [6], which we further indicated in this C57BL6 mouse model as “overgrown brain”. This non-significant observation can be explained because the mice (all male) were grouped in cages that might have caused a ranking hierarchy which we also observed in our study at hepatic steatosis in a similar obese high-fat diet induce C57bl6 mouse model (Figure 2 in reference [6]). Nevertheless the large standard deviation the correlation coefficients between HF-diet obese C57BL6 whole brain fraction (correlation coefficient r2=07.60 in comparison to control chow r2= 0.264), are convincing.
From evolutionary perspective the process of human encephalization -“the mystery of mysteries”- is important through evolutionary time and what was the trigger remains undissolved [15]. In this respect the period of early humans in the Paleolithic pre-agricultural hunter-gatherer society (≈700,000 and 400,000 years ago) suggest periods of insufficient food intake punctuated by the irregular bounty of the kill. The concept of cycles of feast and famine implies the mechanism of the cycling of metabolic processes with the fluxes of feast and famine. During periods of feast individuals with “thrifty” metabolic processes would store more food calories as fat: including –like we hypothetize in this rodent research manuscript- in the human brain. On the other hand, during periods of food shortage, there is a gradual shift in whole body fuel utilization from carbohydrate and fat in the fed state to almost exclusively fat starting already one day of fasting [1]. When glucose availability is low during periods of starvation, the TGs stored in adipose tissue are hydrolyzed to Free Fatty Acids (FFA) and mobilized into plasma to reach the liver [1,3], but also as we demonstrated in this study into the brain (Figure 1). The general perception until presently was that the liver plays a central and pivotal role in these energy conversions and is the major sink of fatty acids in the form of TGs. From here on energy stores in the form of ketone bodies are released to supply vital organs like brain, heart and muscle with sufficient energy [1,3]. This view is generally accepted but this study gives now convincing evidence that Long Chain Fatty Acids (LCFAs) can pass the Blood Brain Barrier (BBB), which is a nearly unexploited research area.
In the past few decades it has become clear that various membrane-associated fatty acid-binding proteins -termed “Fatty Acid TransPorters,” (FATP) for convenience- facilitate the cellular entry of fatty acids, which are then accepted by cytoplasmic Fatty Acid Binding Proteins (FABPc). Furthermore, it has been found that acute changes in fatty acid uptake in response to mechanical (eg, muscle contraction) and hormonal stimuli (insulin) are regulated by specific membrane proteins, in a fashion similar to the regulation of glucose uptake by glucose transporters like the GLUT-transporter family [1]. In the review of [19], these researchers discuss our current understanding of the role of membrane fatty acid transporters in cellular lipid metabolism, focusing on both the acute and chronic regulation of cellular fatty acid uptake and on chronic metabolic diseases, including myocardial disease, insulin resistance, and types 1 and 2 diabetes.
The occurrence of various types of fatty acid transporters (FATPs) -with each displaying a characteristic pattern of tissue distribution- further illustrates their role in cellular lipid homeostasis tuned to the metabolic requirements of a specific tissue. Consequently, it can be concluded LCFAs are an important regulator mechanism of energy for most organisms. They also function on blood hormones, regulating key metabolic functions such as hepatic glucose production. Although LCFAs can diffuse through the hydrophobic core of the plasma membrane into cells, this nonspecific transport cannot account for the high affinity and specific transport of LCFAs exhibited by cells such as cardiac muscle, hepatocytes, and adipocytes. Transport of LCFAs across the plasma membrane is facilitated by a Fatty Acid Transport Protein (FATP), a plasma membrane protein that increases LCFA uptake when expressed in cultured mammalian cells [19]. LCFAs that contain more than 12 carbons, and short- and medium-chain fatty acids originating from the sn-1 and sn-3 positions, undergo different absorption pathways, as will the sn-2 monoacylglycerols (MGs), LCFAs require a protein-mediated process, whilst sn-2 MGs are absorbed by passive diffusion. In vitro studies with adipocytes suggest the possibility that fatty acid transport proteins may have different affinities for different fatty acids, depending on chain length [20]. While the focus of the review of [19] was on heart and skeletal muscle, the concepts outlined for these tissues generally will presumably be applicable to all tissues with an active fatty acid metabolism including brain FATP1. In addition, the latter is also expressed in many tissues, kidney, lung, skin, adipose tissue, heart, and skeletal muscle [19].
Humans represent the positive extreme of all primates, having both a very high quality diet and a large brain that accounts for 20–25% of resting metabolism [15]. So in line with our observations in our research study the primary source for energy should be Very Long Chain Fatty Acids (FLC-FA) obtained from the neutral Triacylglycerol fraction at this obese mouse model with “brain steatosis” [1].
In addition, the brain has a limited ability to synthesize the essential Poly-Unsaturated Fatty Acid (PUFA) docosahexaenoic acid (DHA) from its omega-3 fatty acid precursors. Therefore, to maintain brain concentrations of this PUFA at physiological levels, plasma-derived DHA must be transported across the blood-brain-barriere (BBB). While DHA is able to partition into the luminal membrane of brain endothelial cells, its low aqueous solubility likely limits its cytosolic transfer to the albumin membrane, necessitating the requirement of an intracellular carrier protein to facilitate trafficking of this PUFA across the BBB. This can probably be accomplished because of the intracellular carrier protein fatty acid-binding protein 5(FABP5) which is expressed at the human BBB. The study of [10] demonstrates that the intracellular carrier protein fatty acid-binding protein 5 (FABP5) binds to DHA and is involved in the brain endothelial cell uptake and subsequent BBB transport of DHA, confirming the importance of this cytoplasmic carrier protein in the CNS exposure of this PUFA essential for neuronal function The importance of FA for the developing and adult brain has been recently reviewed [22]. In addition, recent investigations are indicative this might be stimulated by a physiological level of arachidonic acid (ARA) [23] –but probably many other compounds- that could be associated with many different physiological and pathophysiological states. In these studies an increased model of permeability model of the human blood brain barrier (BBB) in the absence of cytokines was suggested [23,24].
We further investigated this topic by trying to find in the literature a correlation between the Body Mass Index (BMI) [3] –a parameter to express the obesity grading- and the head circumference of children newborn. Several studies found the association between maternal BMI and head circumference of newborn: mothers with high BMI head circumference of newborn was significantly higher than in mothers with normal and low BMI [25-29]. These results are indicative that BMI (body mass index) was significantly associated with cerebral index upon adjusting for gender. The strict correlation between BMI and cerebral index suggests that newborn fat deposition may have increased to allow for high myelination in the human brain. The extraordinary fat storage in new-borns would be a consequence of the selection for larger brain size in hominid evolution [29].
Shy in demeanor, though confident of the peculi2021 Copyright OAT. All rights reservrved lipid accumulation during starvation (famine) and a high-fat diet of bovine lard (feast) in our C57bl6 mouse model we hope to contribute by these observations to a valuable contribution to one of the most intriguing and complex questions in biological sciences –what makes us humans? The amount of brain mass exceeding that related to an animal’s body mass is called “encephalization” [15]. However modern analytical laboratory techniques -based on a Systems Biology approach- following modern analytical LCMS-biochemical techniques applying a lipidomics approach [6-12], have never to our awareness been used in human evolution studies. So our observed mechanism of “brain steatosis” in an obese mouse model can be extrapolated towards humans as “trigger” for further evolutionary encephalization studies.
None
- Salway JG (2006) Medical Biochemistry at a Glance ed; ISBN-13:978-1-4051-1322-9(Blackwell publishing ltd)
- van Ginneken VJ (2008) Liver fattening during feast and famine: an evolutionary paradox. Med Hypotheses 70: 924-928. [Crossref]
- van Ginneken VJT (2011) Epidemiology of “Hunger in the World”, the “Hunger- Obesity Paradox”; its Physiological and Endocrine mechanisms; 40 pp. Chapter 4 In: Biology of Starvation in Humans and other organisms. Editor: Todd C. Merkin, ISBN: 978-1-61122546-4, Nova Science Publishers, Inc.; pp. 187-223.
- Bryhn M (2003) Effects of omega-3 fatty acids on mental health. AGROfood industry high-tech. Pronova Biocare AS 420: 1327-1332.
- van Ginneken V, Ham L, de Vries E, Verheij E, van der Greef J, et al. (2016) Comparison of Hormones, Lipoproteins and Substrates in Blood Plasma in a C57bl6 Mouse Strain after Starvation and a High Fat Diet: A Metabolomics Approach. Anat Physiol 6:233.
- van Ginneken V, Verheij E, Hekman M, van der Greef J, Feskens E, et al. (2010) The comparison of lipid profiling in Mouse brain and liver after starvation and a high-fat diet: a Medical Systems Biology approach. Chapter 3 In: Biology of Starvation in Humans and other organisms. Editor: Todd C. Merkin, ISBN: 978-1-61122-546-4, Nova Science Publishers, Inc.; pp. page 151-186.
- van Ginneken V, Verhey E, Poelmann R, Ramakers R, van Dijk KW, et al. (2007) Metabolomics (liver and blood profiling) in a mouse model in response to fasting: a study at hepatic steatosis. Biochimica Biophysica Acta 1771: 1263-1270. [Crossref]
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2016) Metabolomics in Hind Limb and Heart Muscle of a Mouse model after a High-Fat Diet. Anat Physiol 6: 3.
- van Ginneken VJT, Booms R, Verheij E, de Vries E, van der Greef J (2016) The Relation between Non-adipose Muscle Fat and Hepatic Steatosis Studied with Localized 1H Magnetic Resonance Spectroscopy (1H MRS) and LCMS Techniques. Anat Physiol 6: 245.
- van Ginneken V, Verheij E, de Vries E, van der Greef (2016) The discovery of two novel biomarkers in a high-fat diet C56bl6 Obese Model for Non-adipose tissue: a Comprehensive LCMS Study at Hind Limb, Heart, Carcass Muscle, Liver, Brain, Blood Plasma and Food Composition Following a Lipidomics LCMS-Based Approach. Cellular & Mol Med: Open access 2: 2:13.
- van Ginneken V, Verheij E, Hekman M, van der Greef (2017) Characterization of the lipid profile post mortem for Type-2 diabetes in human brain and plasma of the elderly with LCMS-techniques: a descriptive approach of diabetic encephalopathy. Integr Mol Med 4(2): 1-10.
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2017) Potential Biomarkers for “Fatty Liver” (Hepatic Steatosis) and Hepatocellular Carcinoma (HCC) and an explanation of their pathogenesis. Gastroenterol Liver Clin Med 1:1 001.
- Hsu FF, Turk J (2005) Electrospray electrospray ionization with low-energy collisionally activated dissociation Tandem Mass Spectrometry of Complex Lipids: Structural Characterization and mechanisms of fragmentation In: Modern methods for lipid analysis by Liquid Chromatography/Mass Spectrometry and related Techniques WC Byrdwell Editor AOCS Press.
- Field A (2005) Discovering Statistics Using SPSS, Sage Publications ltd, ISBN-0-7619-4451-6, 779 pages.
- Williams MF (2002) Primate encephalization and intelligence. Med Hypotheses 58: 284-290. [Crossref]
- Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thiès F, et al. (2001) Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci 16: 201-204. [Crossref]
- Ebert D, Haller RG, Walton ME (2003) Energy contribution of octanoate to intact rat brain metabolism measured by C13 nuclear magnetic resonance spectroscopy. J Neurosci 23: 5928–5935. [Crossref]
- Marin-Valencia I, Good LB, Ma Q, Malloy CR, Pascual JM (2013) Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J Cereb Blood Flow Metab 33: 175-182. [Crossref]
- Glatz JF, Luiken JJ, Bonen A (2010) Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 90: 367-417. [Crossref]
- Pan Y, Scanlon MJ, Owada Y, Yamamoto Y (2015) Fatty Acid-Binding Protein 5 Facilitates the Blood-Brain Barrier Transport of Docosahexaenoic Acid. Mol Pharm 12: 4375-4385. [Crossref]
- Kurban S, Mehmetoglu I, Yilmaz G (2007) Effect of diet oils on lipid levels of the brain of rats. Indian J Clin Biochem 22: 44-47. [Crossref]
- Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM (2011) Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem 11: 7735-746. [Crossref]
- Murphy EJ (2015) Blood-brain barrier and brain fatty acid uptake: Role of arachidonic acid and PGE2. J Neurochem 135: 845-848. [Crossref]
- Dalvi S, Nguyen HH, On H, Mitchell RW, Aukema HM, et al. (2015) Exogenous arachidonic acid mediates permeability of human brain microvessel endothelial cells through prostaglandin in E2 activation of EP3 and EP4 receptors. J Neurochem 135: 867-879. [Crossref]
- Rezavand N, Seyedzadeh A, Zangeneh M, Veisy F, Rezaie M, et al. (2014) The relationship between maternal pregestational body mass index and head circumference of infants. J Kermanshah University of Med Sci 17: 773–778.
- Nagmoti S, Walvekar P, Mallapur M (2015) Association between body mass index of mother and anthropometry of newborn. Int J Med Res Heal Sci 4: 796–798.
- Kalk P, Guthmann F, Krause K, Relle K, Godes M, et al. (2009) Impact of maternal body mass index on neonatal outcome. Eur J Med Res 14: 216-222. [Crossref]
- Geraedts EJ, van Dommelen P, Caliebe J, Visser R, Ranke MB, et al. (2011) Association between head circumference and body size. Horm Res Paediatr 75: 213-219. [Crossref]
- Bayat PD, Ghanbari A, Chehreie S (2010) Relationship between body mass index and the development of cranium in Arak newborns (central Iran) Ital J Anat Embryol 115: 218-222. [Crossref]


