Abstract
With the advent of technology that can reliably detect them, interest in circulating tumor cells have escalated with over 18,000 publications and its involvement in 911 trials. Its presence in the blood stream connotes more aggressive and serious disease. This has led to a revision in the American Joint Commission on Cancer TNM classification for breast cancer. Presence of circulating tumor cells upcodes presumed non-metastatic breast cancer (M0) to an intermediate stage M0(i+). This review will describe the methods to capture and detect circulating tumor cells with a focus on the most analytically valid system – CellSearch. The studies underpinning the established uses of CTC enumeration in prognosis of metastatic cancers (breast, prostate and colon) will be highlighted as well as the imminent deployment in early breast cancer. Emerging applications in monitoring cancer treatment and promising indications in other cancers will also be examined.
Key words
circulating tumor cells, CellSearch, clinical utility
Introduction
Most cancer morbidity and mortality can be ascribed to metastatic disease. Consequently, cancer patients are monitored for metastatic disease by periodic surveillance using clinical findings, biomarkers, and imaging. Biomarkers are indicators of normal or pathologic biological processes. They also reflect response to therapy. The major route to distant metastasis is when cancer cells traverse the blood stream. These cells have been named circulating tumor cells (CTCs). Like other cancer biomarkers, CTCs provide a measure of disease burden as well as cancer progression or improvement. However, the miniscule quantity of CTC in the bloodstream amidst a sea of blood cells (white, red and platelets) renders their detection challenging. CTCs have gained prominence and interest in recent years with the advent of technology that can reliably detect them. Additional developments have allowed CTCs to be characterised. The great interest in CTCs is reflected by the volume of recent published literature. A search of PubMed (accessed December 2016) using the keywords “circulating tumor cells” revealed over 18,000 publications (Figure 1). A corresponding search for “circulating tumor cells” in the NIH clinical trials database (accessed December 2016) uncovered 911 studies; the distribution by cancer type is provided in Figure 2.
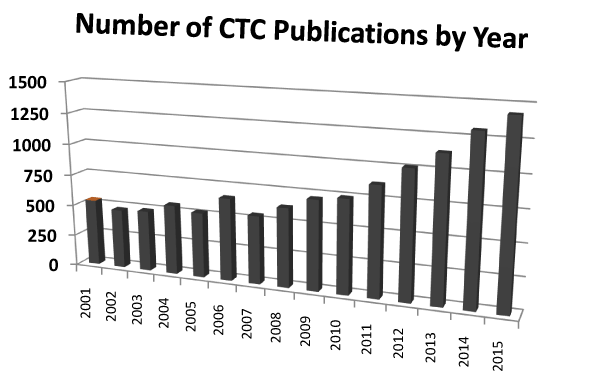
Figure 1. Annual number of CTC publications in PubMed from 2001-2015.
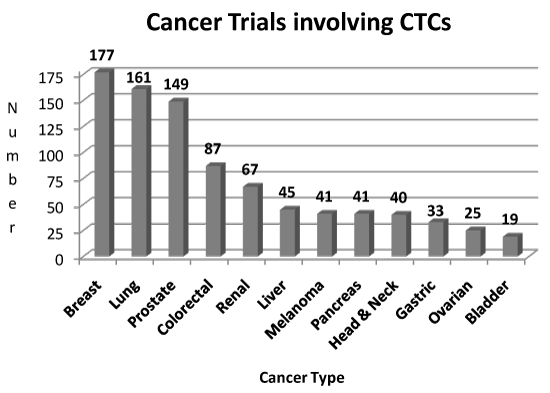
Figure 2. Clinical trials involving CTCs in the last 15 years by type at clinicaltrials.gov.
In the 2007 American Society of Clinical Oncology (ASCO) recommendations on the use of on tumor markers for breast cancer (BC), CTCs were cited [1]. A new M0(i+) category was introduced for the TNM (tumor, node, metastasis) staging of BC [2]. This category, in a patient without symptoms or signs (clinical or radiographic) of metastases, manifests as deposits of molecularly or microscopically detected tumor cells (no larger than 0.2 mm) in blood, bone marrow, or other non-regional nodal tissue. Thus, in patients with presumed non-metastatic breast cancer (M0), presence of CTCs in the blood renders them in a higher stage – intermediate between M0 & M1. This development has yet to diffuse into the practice of oncopathology and its clinical and therapeutic implications are awaited.
As a newer biomarker, CTCs are gaining greater utility. This review will briefly describe the methods used to isolate and detect CTCs in section 2. Section 3 will highlight the established uses of CTC enumeration in prognosis of metastatic cancers (breast, prostate and colon), imminent deployment in prognosis of early/non-metastatic tumors, emerging applications in monitoring cancer treatment, and promising indications in other cancers. Potential and future use of CTCs will be elaborated in Section 4.
Methods for CTC Capture and Detection
An early publication demonstrated that when blood from cancer patients are immuno-magnetically enriched, CTCs can be detected by immunofluorescence and flow cytometry [3]. In the recent literature from 2010-2015, over 35 different CTC methods were identified [4]. More new devices have been recently described [5-9]. CTC detection modalities have been well documented [10-12] and will not be covered in this review.
Among the many CTC technologies only CellSearch (Janssen Diagnostics, Raritan, NJ, USA) is FDA-approved. As the most studied technique it merits some attention. The assay allows the capture and enumeration of tumor cells circulating in peripheral blood in a standardized and almost fully automated format [13-15]. The CellSearch system comprises an automated CellTracks AutoPrep instrument to capture and label the CTCs and a semi-automated immuno-fluorescent microscope (CellTracks Analyzer) for cell detection. Blood, collected in proprietary tubes, are stable for up to 96 hours prior to analysis. Buffer containing ferrofluid (nanoparticles with a magnetic core and an outer layer coated with antibodies to Epithelial Cell Adhesion Molecules (EpCAM-Ab) is added to the sample. Following immuno-magnetic capture and enrichment, CTCs are permeabilized before exposure to fluorescent antibodies directed against the cytoplasm (anti-CK-8,18,19), nucleus (DAPI) and leucocytes (anti-CD45). Thereafter the mixture is transferred into a plastic cartridge surrounded by a magnetic sheath. The magnet attracts the CTCs to the surface of the cartridge forming a thin layer of cells that are amenable to imaging. The analyzer can accommodate up to 8 samples in each run of 2-3 hours. Each assay utilizes a control sample comprising SK-BR3 breast cancer cell lines tagged with 2 different fluorescent labels one each for a population of low CTC count (approximately 40-50 cells) and another for high CTC count (approximately 1000 cells). Following cell capture, the samples are transferred singly to the Analyzer which scans the surface of the cartridge. Fluorescent objects are imaged and displayed in a gallery for classification. CTCs are defined as CK+, DAPI+,CD45- fluorescent objects of at least 4 microns and with co-location of cytoplasmic and nuclear images. The number of cells present is expressed as CTCs per 7.5 mL of blood analyzed. The 96-hour limit from sample draw to analysis is a major hurdle for multi-center studies requiring sample transport. This setback may now be overcome by using a recently described cell transport solution that renders CTCs stable for up to a week [16]. Almost all healthy subjects have no CTCs by CellSearch while 36% of 964 patients with metastatic cancers had > 2 CTCs [13]. This was also established in a local study of 101 healthy multi-ethnic Asian men and women [17]. The performance of the CellSearch system has been verified [17-21] and includes an external quality assurance scheme [22] that is now available through the American Association of Bioanalysts. The analytical validity of the CellSearch system is secure.
Various technologies employing different enrichment and detection methods may not always detect the same CTC populations. In metastatic BC the concordance for CTC detection using the CellSearch and AdnaTest CTC assays was 64% in one study [23] but other studies have found that they were equivalent [24], CellSearch superior [25], or complementary and best used in combination [26]. AdnaTest (AdnaGen) employs antibody-coated magnetic beads against EpCAM and MUC1 (a breast cancer antigen) for CTC capture followed by RT-PCR for detection of the tumor transcripts. There are few head to head comparisons of different CTC platforms. Reports of EpCAM-negative CTCs [27-28] raised the spectre of CTC underestimation by CellSearch [29]. However, EpCAM-negative CTCs have since been shown to be unrelated to poor outcomes in metastatic lung cancer [30]. This issue merits more extensive study.
The CellSearch system is found in all major reference laboratories in the US including Quest Diagnostics, LabCorp and ARUP. Sixteen of the top 20 US academic medical centers including Mayo have the CellSearch System for the management of metastatic breast, colon, and prostate cancer. In the US, the CellSearch CTC test has been assigned an American Medical Association Category 1 Current Procedural Terminology (CPT) Code 86152 (CTC testing) and 86153 (CTC interpretation) for re-imbursement from the Center for Medicare & Medicaid Services (CMS) and healthcare insurers. Category 1 codes are only assigned to services and procedures whose clinical efficacy has been well established and documented in peer-reviewed literature.
Utility of CTCs
Clinical trials have shown that CTC testing offers useful information for the physician to monitor progression of disease or response to treatment. Studies have also demonstrated that detection and quantitation of CTC at any time during the course of disease is an independent predictor of progression-free survival (PFS) and overall survival (OS) in patients with metastatic cancers (breast, prostate, and colon) and provides valuable prognostic information sooner to support patient care decisions. In this review, publications supporting the clinical validity of CTCs especially with the CellSearch system will be highlighted since it has the best analytical validity.
Seminal studies formed the basis for approval of CellSearch CTC in patients with metastatic cancers (breast, prostate, and colon) [31-33] from the US FDA. Cristofanilli found that in 49% of 177 patients with metastatic breast cancer (BC) had 5 or more CTCs per 7.5 ml of blood prior to commencing treatment [31]. This CTC cut-off was an independent predictor of PFS (7.0 versus 2.7 months) and OS (> 18.0 versus 10.1 months). Information arising from this trial led to FDA clearance of the CellSearch assay for metastatic BC patients in 2004.
A multicenter, prospective clinical trial (n = 231) conducted between 2004 to 2006 revealed that the number of CTCs predicted disease progression and OS [32] in patients with hormone-resistant, androgen-independent, or castration-resistant prostate cancer (PC). CTC counts and PSA were assessed every 4-6 weeks. The favorable group (n = 94) consisted of patients with baseline CTCs below 5 per 7.5 mL while those with ≥ 5 CTCs formed the unfavorable group (n = 125). While median PFS was slightly longer for the favourable group compared to the unfavorable group (5.8 versus 4.3 months), median OS was significantly longer (21.7 versus 11.5 months). The median OS in patients whose baseline CTCs improved with treatment were far greater than those whose CTCs progressed (21.3 versus 9.3 months). This trial results formed the basis for FDA’s approval of the CellSearch assay for metastatic PC patients in 2008.
Between 2004 and 2006 another multicenter, prospective clinical trial (n = 430) in metastatic colorectal cancer (CRC) demonstrated that CTCs could predict CRC disease progression and survival [33,34]. Imaging (CT or MRI of the chest, abdomen, and pelvis) and CTC counts (baseline and every 3-4 weeks) were determined for each patient. Patients with baseline CTC count below 3 constituted the favourable group (n = 305) while those with a cell count ≥ 3 formed the unfavourable group (n = 108). PFS for the favourable group were significantly higher compared to the unfavourable group (7.9 versus 4.5 months) while median OS was longer (18.5 versus 9.4 months). This data supported the FDA clearance of the CellSearch assay for metastatic CRC patients in 2007.
Established uses of CTCs as a Prognostic Biomarker
The prognostic utility of CTCs for metastatic breast, prostate and colorectal cancers is firmly established and continues to be confirmed in several recent studies. In the aggregate, they constitute clinical validity.
Metastatic Breast Cancer: CTC counts ≥ 5 cells and rising in patients with metastatic BC have superior and independent prognostic value compared to standard determinations (clinical and imaging) [35-39]. During therapy, CTC counts associates with progression and mortality [35]. Notably patients whose baseline CTCs improved at follow-up fared better (median OS 19.8 months) than those whose CTCs progressed (median OS 10.6 months).
In a landmark study [39] CellSearch CTCs strongly correlated with radiographic disease progression in metastatic BC patients (n=68) receiving endocrine or chemotherapy. Patients with ≥ 5 CTCs had 6.3 times the odds of radiographic disease progression compared to those with < 5 CTCs. The study supports the use of CTC as an adjunct to standard clinical and imaging assessments. The authors also suggest that as CTCs are subject to less observer variability than imaging, its use may also reduce the number of follow-up radiologic studies needed.
The first comprehensive meta-analysis of CTCs in metastatic BC (n=3069) in 2012 concluded that CTCs are reliable prognostic factors [40] for PFS (12 studies, hazard ratio 1.78) and OS (19 studies, hazard ratio 2.33). Multiple trials have since supported the prognostic significance of CTCs for PFS and OS [25,41-45].
In a pooled analysis of MBC (n = 1944) in 2014, elevated CTC counts at baseline and 1 month after treatment associates with a 2-fold decrease in PFS and OS [46]. Tumor markers (CEA and CA15-3) did not add to prognostic significance. CTC’s prognostic value was consistent across all subtypes of disease, irrespective of receptor status (hormone or Her-2), number or nature of metastases, and type or line of therapy. In multi-variate analysis, CTC count was the strongest prognosticator for PFS and OS. This study provides level-one evidence for clinical validity of CTC as adverse prognostic markers in metastatic BC [4].
The recent SWOG S0500 trial (n = 595), strongly validates CTC’s prognostic utility both at baseline and at 1 month after treatment [47]. Those with no CTCs at baseline (n = 276) fared the best (median OS 35 months) while those whose CTCs improved after 1 month of chemotherapy (n = 165) had better OS (23 months) than those with elevated CTCs (n = 123, median OS 13 months). Similar results were reported in another study (n = 393) [45]. In 300 Chinese MBC patients [48], CTCs provided substantial prognostic information and is independently associated with PFS and OS.
The cut-off of > 5 CTC employed in many previous studies has been verified [49]. Additionally, early changes of CTC counts correlated with treatment outcomes. A distinct aggressive subtype of BC (10-20% of all cases) is the triple-negative breast cancer (TNBC). In a meta-analysis (n = 642) CTCs predicted TNBC progression [50].
The data on the adverse prognostic value of CTC in metastatic BC is well established.
Metastatic Prostate Cancer: CTCs are now included among prostate biomarkers beyond prostate-specific antigen (PSA) [51,52].
The CellSearch CTC cut-off of 5/7.5 mL blood has been verified as a prognostic marker for metastatic PC [53,54]. CTC counts correlate with disease outcome [55,56]. In patients receiving docetaxel [32,57] or abiraterone [58] baseline CTCs were prognostic for OS and poorer standard assessments (PSA and imaging). Moreover, OS is reduced when CTCs increase during treatment [56]. CTC levels are also more prognostic for OS than PSA reductions [32,57]. CTC reductions during treatment are also prognostic [59]. The relation between outcomes and CTCs is stronger when CTCs are used as a continuous variable rather than a fixed cut-point [56].
The correlation between CTC, PSA, Gleason score, and TNM stage in patients with metastatic castration-resistant prostate cancer (mCRPC) was examined; CTCs correctly staged prostate cancer and assessed prognosis of mCRPC [60]. Patients with ≥ 4 CTCs/7.5 mL had a significantly shorter median OS and PFS (24 versus 45 months and 7.0 versus 44 months, respectively). The risk of mortality and progression for patients with ≥ 4 CTCs was 4.1 and 8.5 times greater respectively. CTC of ≥ 4 was an independent prognostic factor for PFS (hazard ratio 5.9).
CTCs were prognostic in a phase III abiraterone trial (n = 711) in mCRPC previously treated with docetaxel [61]. A biomarker panel containing CTC and LDH was a surrogate for overall 2-year survival. The OS in low-risk patients (CTCs < 5) versus high-risk subjects (CTCs ≥ 5 and LDH > 250 U/L) at 12 weeks was 46% and 2%, respectively.
CTC counts appear earlier and better predict survival/treatment response in mCRPC than current approaches [62]. Using the CellSearch system (n = 122) baseline CTC counts (≥ 5) predicted OS after 1, 4, or 10 cycles of therapy. Early CTC counts after cycle 1 was comparable to standard assessment methods after cycle 4 of treatment.
CTCs (>5) also identify a more aggressive phenotype than standard methods (PSA, LDH, alkaline phosphatase, Hb) in mCRPC [63]. The hazard ratio for death in those with normal versus elevated CTCs was 0.43.
The studies on the prognostic value of CTC in metastatic PC are firmly grounded.
Metastatic Colorectal Cancer (CRC): The use of CTCs in metastatic CRC has been reviewed [64-66]. In a 2015 meta-analysis, the prognostic utility of CTC (> 3) for metastatic and non-metastatic CRC (n = 1847; 11 studies) has been affirmed [67]. Patients with high CTCs had poorer PFS and OS. Patients who converted from low to high CTC or had persistently high CTC experienced worse outcomes than those whose CTC counts improved. CTC counts were also higher in CRC with hepatic metastasis.
In metastatic CRC (n=217), subjects with high CEA (> 25ng/mL) and high CTC had lower survival (11.7 versus 20.8 months) [68]. CTCs provided added prognostic information at 3-5 weeks and 6-12week time points regardless of CEA levels.
CTCs before and during treatment showed a strong prognostic correlation (odds ratio 5.5 to 14.0) with radiographic disease progression at 6 months in patients (n = 60) receiving chemotherapy for CRC [69]. Patients with baseline CTC positivity had shorter PFS (181 versus 329 days). Thus, CTCs are complementary tools for outcomes prediction.
In another meta-analysis (12 studies, n = 1329), CTCs were prognostic in resectable colorectal cancer with liver metastases or widespread metastases [70]. OS and PFS were worse in those with CTC positivity (hazard ratio 2.47 and 2.07 respectively). In the studies with multivariate analysis (n = 8) CTC was an independent prognostic factor for survival. Presence of CTCs after surgical resection are adverse prognostic factors for stage I-III CRC [71] and stage II/III CRC [72]. In mCRC subjects without any increase in CEA or other markers, CTCs may allow more efficient disease monitoring [73].
Investigations on the prognostic value of CTC in metastatic CRC are secure.
Imminent Use of CTCs
CTCs are prognostic in early or non-metastatic breast cancer (nMBC).
Early Breast Cancer (BC) Prognosis: In presumed early BC or nMBC patients, tumor cells had been detected in the bone marrow using laborious in-house methods. Evaluation of early BC patients often did not include assessment for such micro-metastasis or disseminated tumor cells (DTC) because of analytical tedium and invasive sample collection. Nonetheless, concordance between CTCs and presence of DTCs in bone marrow is between 66-94% and their adverse prognostic impact has been reviewed [74]. Besides, the 2010 TNM classification [2] tacitly accorded the prognostic impact of DTC and CTC a level 1 evidence when it introduced the cM0(i+) category for BC patients without clinical or imaging evidence of metastasis but with micrometastasis (< 0.2 mm size or molecularly detected). The trial of trastuzumab and CTC detection in M0(i+) nmBC is currently underway in Europe [74].
In a 2012 meta-analysis of the 1990-2012 literature, CTCs were prognostic for PFS (19 studies, hazard ratio 2.86) and OS (13 studies, hazard ratio 2.78) in nMBC [40].
The prognostic significance of CTCs in newly diagnosed breast cancer was affirmed in a prospective evaluation of women with stage I-III breast cancer (n = 302) followed up to 35 months [75]. CTCs were tested prior to surgery and adjuvant chemotherapy. In 24% of patients (n=73) presence of CTCs was associated with a greater risk of disease progression (hazard ratio 4.62) and mortality (hazard ratio 4.04). This was not predicted by primary tumor characteristics. Besides CTCs did not correlate with axillary nodal status or other clinical or pathologic factors. CTCs before and 1-2 years after surgery was also reported to predict shorter PFS and OS for stage I-III breast cancer (n = 403) [76].
In a 2014 study of early average-to-high risk BC before (n = 2000) and after (n = 1500) chemotherapy, pre-treatment CTCs associates with shorter PFS (hazard ratio 2.11) and OS (hazard ratio 2.18) [77]. At all cut-off levels, CTCs exerted a significant impact on outcomes. CTC ≥ 5 connotes the highest risk for relapse.
In the largest pooled analysis of early BC (n = 3173), CTC was an independent predictor of poor PFS (hazard ratio 1.89) and OS (hazard ratio 1.97) [78]. CTCs were detected in 20.2% of cases and these tumors tended to be larger, with higher histologic grade and greater nodal involvement. The prognostic relevance of CTCs in early BC has been affirmed in very recent reviews [74,79].
Nearly all trials have reported a strong correlation between CTCs and survival. The data on the prognostic value of CTCs in early nmBC is solid and clinically valid.
Emerging Applications for CTCs – Therapy Monitoring
CTCs may be used to stratify patients and treatment response. Based on CTC counts during treatment patients may be assigned to different prognostic groupings:
- Low or undetectable CTCs – longest PFS and OS
- High baseline CTCs which decline with therapy – intermediate PFS and OS
- Persistently elevated CTCs – shortest PFS and OS
CTC persistence affects survival [74]. CTC clearance can be used as a ‘‘surrogate’’ for improved survival. In fact, some oncologists consider presence of CTCs after the first cycles of therapy as therapeutic futility [80].
Breast Cancer: Elevated CTC counts (> 5) are associated with adverse survival. In a 2006 MBC study [35] (n = 177), four measurements of CTC at monthly intervals after commencing therapy showed that elevated CTCs at any time during treatment indicates subsequent rapid disease progression and mortality. The same group compared imaging studies in 138 subjects before and 10 weeks after starting therapy versus monthly CTC counts [36]. In patients with radiologic progression (n = 44), OS was significantly shorter in those with elevated CTCs (6.4 versus 19.6 months). Even in patients with radiologic nonprogression (n=96), OS was significantly shorter in those with elevated CTCs (15.3 versus 26.9 months). Thus, CTC is an earlier, more reproducible indication of disease status than current imaging methods and may be a superior surrogate end point.
In an MBC trial (n = 393), CTCs were tested at baseline (BL) and after 1 cycle of a new line of treatment (1c). Unfavorable BL and 1c CTC profiles were associated with progressive disease and lower survival [44]; where BL CTC improved outcomes were favourable.
In the SWOG S0500 phase III MBC study [47], CTC-positive patients (n = 123) despite chemotherapy (group C) showed shorter median OS (13 months) compared to those with low initial CTC counts (group A, n = 76) and those with decreased CTCs after the first treatment cycle (group B, n = 165). Early chemotherapy change in Group C after one treatment cycle did not improve survival, implying that more effective treatment is needed in this high-risk cohort. Persistence of CTCs identifies a higher risk group who are not responding to therapy and may require consideration of alternative treatment strategies.
In average to high-risk nMBC, CTCs were found to have independent prognostic relevance both before and after adjuvant chemotherapy (n = 2026) [77]. The hazard ratios for survival were 2.11 (PFS) and 2.18 (OS) respectively. The prognosis was worst in patients with at least five CTCs per 30 mL blood. Persistence of CTCs after chemotherapy showed a negative influence on PFS and OS (hazard ratio 1.12 and 1.16 respectively). In a smaller study (n = 57) of non-metastatic TNBC, CTC were assessed after completion of neoadjuvant chemotherapy. Persistence of CTCs was an independent predictor of relapse and survival; hazard ratios for PFS and OS were 5.25 and 7.04 respectively [81].
In another nMBC trial [82] (n = 237), taxane-based chemotherapy resulted in a higher incidence of CTCs' elimination than taxane-free regimens (49.7% versus 33.0%) in those with detectable CTCs before chemotherapy. Taxane-based adjuvant treatment in these CTC-positive patients had more favourable PFS than the taxane-free group after a median follow-up of 71 months and is reflected in the shift towards CTC-negative status.
Prostate Cancer: CTC-counts provide early prediction of treatment efficacy and optimized sequential treatment [62]. In the COU-301 abiraterone trial [61], post-treatment CTC was a surrogate for OS. A composite biomarker panel, comprising CTC (favorable vs. unfavorable) and lactate dehydrogenase (LDH: > 250 IU/L vs. < 250 IU/L) 12 weeks after treatment initiation, stratified patients into good (CTC < 5 and LDH < 250), intermediate (CTC < 5 and LDH > 250), and poor (CTC > 5) risk groups. CTCs combined with PSA velocity may also offer insights into the prognosis and management of advanced prostate cancer [83].
Currently we cannot easily differentiate local from distant recurrent prostate cancer. CTCs may be a surrogate indicator for whether or not to offer systemic or local treatment [84].
CTCs can be detected in early-stage prostate cancer patients receiving salvage radiotherapy and post-treatment reduction in CTC may may indicate response to radiation therapy [85].
Recent CRPC trials have incorporated CTC detection, besides imaging and patient-reported outcomes, in order to improve drug development [86].
In CRPC with bone metastases, CTC counts helped assess response and predicted outcomes in docetaxel therapy [87]. CTC guidance for prostate cancer treatment has been elegantly summarised [88,89].
Colorectal Cancer: CTCs in mesenteric veins of patients with mCRC [90] signify locally advanced disease. In resectable colorectal liver metastases or widespread metastatic CRC, CTCs are associated with disease progression and poor survival [70]. CTCs may predict CRC recurrence/progression [91] and help select high-risk stage II patients for adjuvant chemotherapy [92]. CTCs have also been used as an outcome marker after chemotherapy [93].
Promising Uses for CTCs in Oncology
New research has revealed CTC’s potential in other cancers such as ovary, liver, pancreas, head and neck, melanoma, esophagus, gastric, lung, bladder, CNS, and cancers of unknown origin [94-128]. Developments in lung and renal cancers will be briefly surveyed here [94-128].
Lung Cancer: Lung cancer, comprising small cell lung cancer (SCLC, 13%) and non-small cell lung cancer (NSCLC 85%), are major causes of death. They often present with extensive local disease or distant spread. CTCS have been used to evaluate recurrence detection, prognosis, and treatment response in both SCLC and NSCLC. In diagnosed or suspected lung cancer (n = 150), a correlation between CTC count and extent of disease as patients’ condition progressed was found consistent with results seen in other cancer subtypes [129]; 30.6% of lung cancer patients had identifiable CTCs. Most of the patients in this study were early stage cancer, with 110 patients out of 150 Stage I–IIIA.
CTCs are often detected in very large numbers (86%) in SCLC [130] (n=50). The median CTC count was 28 per 7.5 ml of blood, with a range from 0 to 44,896 cells per 7.5 ml. High numbers of CTCs correlated with poor survival. CTC count > 300 and CTC count < 2 had median survivals of 4.5 months and 14.8 months respectively. In addition, a persistently elevated CTC count after one cycle of chemotherapy was a strong indicator of adverse prognosis.
The same group reported on CTCs in Stage III and IV NSCLC [131], both before and after administration of one cycle of chemotherapy. CTC numbers correlated with stage, with more CTCs detected in Stage IV patients than in Stage III; 32% of Stage IV patients, 7% of Stage IIIb patients and 0% of Stage IIIa patients had ≥ 2 CTCs. Both PFS and OS correlated with CTC numbers. A CTC count ≥ 5 was found to be a negative prognostic indicator. PFS of 6.8 vs. 2.4 months, and OS of 8.1 vs. 4.3 months were observed in patients with less than 5 CTCs compared to those with 5 or more CTCs. In addition, an exploratory analysis of the change in CTC count observed after one cycle of chemotherapy was predictive for PFS and OS, with PFS of 5.4 vs. 1.9 months and OS of 8.3 vs. 3.3 months for patients who experienced a decrease in CTC number compared to those with CTC increase. Of interest is the marked difference in CTC levels detected in NSCLC and SCLC, as compared using the same technique by the same group; much larger numbers of CTCs are seen in SCLC than in NSCLC. In advanced NSCLC [132] CTCs (> 1) were detected in 78% of 41 patients. Decreases in CTC counts was associated with radiographic response by FDG-PET and longer PFS.
The utility of circulating tumor cells in lung cancer has been recently reviewed [133,134].
Renal Cancer: Studies of CTCs in RCC are limited but slowly increasing. Presence of CTCs were first reported in RCC in the 1990s [135]. In small studies, CTCs were found in 25-92% of patients [13,136,137]. In the largest RCC study [138] (n = 154) CTCs were present in 52.6% (81/154) of subjects. CTC was an independent prognostic factor and also correlated with involvement of lymph nodes. More and larger studies of CTCs in RCC are needed.
Conclusion
After 20 years of research and over 18,000 publications, CTCs have emerged as an integral part of the clinical armamentarium in oncology. CTCs play a significant part in the metastatic disease process and our understanding of their relevance is growing. The ability to capture and enumerate CTCs constitutes an increasing role in the management of patients with metastatic breast, colorectal, and prostate cancer. It can assist in clinical decision-making and constitutes additional complementary information to guide prognosis and treatment. Before commencing treatment, the presence and number of CTCs should be used to assess prognosis. After the first cycle of therapy, CTCs should be used to assess treatment efficacy [139]. The analytical and clinical validity of CTCs is beyond reproach. Data on its clinical utility is accumulating. CTCs outperform current standard tools like serum tumor markers and imaging. Just like these diagnostic markers, persistently elevated CTCs can only indicate that the current treatment is no longer effective but not provide an alternate therapeutic strategy. Certainly, CTCs will gain insights from molecular biology and genetics to improve and refine its utility. While genomic and molecular profiling of CTCs is an active area of current research [4] its clinical application is awaited. All clinicians and scientists need to be mindful of the complementarity of information provided by different investigative modalities and all of them should be used for maximum patient benefit.
References
- Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, et al. (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25: 5287-5312. [Crossref]
- American Joint Committee on Cancer: Breast (2010) AJCC Cancer Staging Manual. 7th edition. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL (Eds), New York pp: 347-376.
- Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, et al. (1998) Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A 95: 4589-4594. [Crossref]
- Ignatiadis M, Lee M, Jeffrey SS (2015) Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin Cancer Res 21: 4786-4800. [Crossref]
- Chen JF, Zhu Y, Lu YT, Hodara E, Hou S, et al. (2016) Clinical Applications of NanoVelcro Rare-Cell Assays for Detection and Characterization of Circulating Tumor Cells. Theranostics 6: 1425-1439. [Crossref]
- Dhar M, Pao E, Renier C, Go DE, Che J, et al. (2016) Label-free enumeration, collection and downstream cytological and cytogenetic analysis of circulating tumor cells. Sci Rep 6: 35474. [Crossref]
- Gogoi P, Sepehri S, Zhou Y, Gorin MA, Paolillo C, et al. (2016) Development of an automated and sensitive microfluidic device for capturing and characterizing circulating tumor cells (CTCs) from clinical blood samples. PLoS One 11: e0147400. [Crossref]
- Theil G, Fischer K, Weber E, Medek R, Hoda R, et al. (2016) The use of a new Cell Collector to isolate circulating tumor cells from the blood of patients with different stages of prostate cancer and clinical outcomes - a proof-of-concept study. PLoS One 11: e0158354. [Crossref]
- Yeo T, Tan SJ, Lim CL, Lau DP, Chua YW, et al. (2016) Microfluidic enrichment for the single cell analysis of circulating tumor cells. Sci Rep 6: 22076. [Crossref]
- Ferreira MM, Ramani VC, Jeffrey SS (2016) Circulating tumour cell technologies. Mol Oncol 10: 374-394. [Crossref]
- Paoletti C, Hayes DF (2016) Circulating Tumor Cells. Adv Exp Med Biol 882: 235-258. [Crossref]
- Zhang J, Chen K, Fan ZH (2016) Circulating Tumor Cell Isolation and Analysis. Adv Clin Chem 75: 1-31. [Crossref]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, et al. (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with non-malignant diseases. Clin Cancer Res 10: 6897-6904. [Crossref]
- de Wit S, van Dalum G, Terstappen LW (2014) Detection of circulating tumor cells. Scientifica (Cairo): 819362. [Crossref]
- Coumans F, Terstappen L (2015) Detection and Characterization of Circulating Tumor Cells by the CellSearch Approach. Methods Mol Biol 1347: 263-278. [Crossref]
- Stefansson S, Adams DL, Ershler WB, Huyen Le, and David H (2016) A cell transportation solution that preserves live circulating tumor cells in patient blood samples. BMC Cancer 16: 300. [Crossref]
- Aw TC, Goh M, Swe S (2014) Experience with the use of the CellSearch system for enumeration of circulating tumor cells (CTCs) in Asian subjects. Clin Chem 10(Suppl): S5-S6.
- Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, et al. (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 13: 920-928. [Crossref]
- Cummings J, Morris K, Zhou C, Sloane R, Lancashire M, et al. (2013) Method validation of circulating tumour cell enumeration at low cell counts. BMC Cancer 13: 415. [Crossref]
- Ignatiadis M, Riethdorf S, Bidard FC, Vaucher I, Khazour M, et al. (2014) International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res 16: R43. [Crossref]
- Wang L, Balasubramanian P, Chen AP, Kummar S, Evrard YA, et al. (2016) Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin Oncol 43: 464-475. [Crossref]
- Kraan J, Sleijfer S, Strijbos MH, Ignatiadis M, Peeters D, et al. (2011) External quality assurance of circulating tumor cell enumeration using the CellSearch(®) system: a feasibility study. Cytometry B Clin Cytom 80: 112-118. [Crossref]
- Fehm T, Müller V, Aktas B, Janni W, Schneeweiss A, et al. (2010) HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 124: 403-412. [Crossref]
- Andreopoulou E, Yang LY, Rangel KM, Reuben JM, Hsu L, et al. (2012) Comparison of assay methods for detection of circulating tumor cells in metastatic breast cancer: AdnaGen AdnaTest BreastCancer Select/Detect versus Veridex CellSearch system. Int J Cancer 130: 1590-1597. [Crossref]
- Muller V, Riethdorf S, Rack B, Janni W, Fasching PA, et al. (2012) Prognostic impact of circulating tumor cells assessed with the CellSearch system and AdnaTest Breast in metastatic breast cancer patients: the DETECT study. Breast Cancer Res 14: R118. [Crossref]
- Gorges TM, Stein A, Quidde J, Hauch S, Röck K, et al. (2016) Improved detection of circulating tumor cells in metastatic colorectal cancer by the combination of the CellSearch system and the AdnaTest. PLoS One 11: e0155126. [Crossref]
- Königsberg R, Obermayr E, Bises G, Pfeiler G, Gneist M, et al. (2011) Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncol 50: 700-710. [Crossref]
- Mikolajczyk SD, Millar LS, Tsinberg P, Coutt SM, Zomorrodi M, et al. (2011) Detection of EpCAM-negative and cytokeratin-negative circulating tumor cells in peripheral blood. J Oncol: 252361.
- Grover PA, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE (2014) Circulating tumor cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol 25: 1505-1516. [Crossref]
- de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, et al. (2015) The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci Rep 5: 12270. [Crossref]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, et al. (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351: 781-791. [Crossref]
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, et al. (2008) Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 14: 6302-6309. [Crossref]
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. (2008) Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26: 3213-3221. [Crossref]
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. (2009) Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 20: 1223-1229. [Crossref]
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, et al. (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12: 4218. [Crossref]
- Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, et al. (2006) Circulating tumor cells versus imaging - predicting overall survival in metastatic breast cancer. Clin Cancer Res 12: 6403. [Crossref]
- Cristofanilli M, Broglio KR, Guarneri V, Jackson S, Fritsche HA, et al. (2007) Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer 7: 471-479. [Crossref]
- Dawood S, Broglio K, Valero V, Reuben J, Handy B, et al. (2008) Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer 113: 2422-2430. [Crossref]
- Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, et al. (2009) Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 27: 5153-5159. [Crossref]
- Zhang L, Riethdorf S, Wu G, Wang T, Yang K, et al. (2012) Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res 18: 5701-5710. [Crossref]
- Pierga JY, Hajage D, Bachelot T, Delaloge S, Brain E, et al. (2012) High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol 23: 618-624. [Crossref]
- Giordano A, Cristofanilli M (2012) CTCs in metastatic breast cancer. Recent Results Cancer Res 195: 193-201. [Crossref]
- Giordano A, Egleston BL, Hajage D, Bland J, Hortobagyi GN, et al. (2013) Establishment and validation of circulating tumor cell-based prognostic nomograms in first-line metastatic breast cancer patients. Clin Cancer Res 19: 1596-1602. [Crossref]
- Wallwiener M, Hartkopf AD, Baccelli I, Riethdorf S, Schott S, et al. (2013) The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res Treat 137: 503-510. [Crossref]
- Wallwiener M, Rieth2021 Copyright OAT. All rights reservJ, et al. (2014) Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer 14: 1-12. [Crossref]
- Bidard FC, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, et al. (2014) Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 15: 406-414. [Crossref]
- Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, et al. (2014) Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 32: 3483-3489. [Crossref]
- Jiang ZF, Cristofanilli M, Shao ZM, Tong ZS, Song EW, et al. (2013) Circulating tumor cells predict progression-free and overall survival in Chinese patients with metastatic breast cancer, HER2-positive or triple-negative (CBCSG004): a multicenter, double-blind, prospective trial. Ann Oncol 24: 2766-2772. [Crossref]
- Helissey C, Berger F, Cottu P, Diéras V, Mignot L, et al. (2015) Circulating tumor cell thresholds and survival scores in advanced metastatic breast cancer: the observational step of the CirCe01 phase III trial. Cancer Lett 360: 213-218. [Crossref]
- Lu YJ, Wang P, Wang X, Peng J, Zhu YW, et al. (2016) The significant prognostic value of circulating tumor cells in triple-negative breast cancer: a meta-analysis. Oncotarget 7: 37361-37369. [Crossref]
- Saini S (2016) PSA and beyond: alternative prostate cancer biomarkers. Cell Oncol (Dordr) 39: 97-106. [Crossref]
- León-Mateos L, Vieito M, Anido U, López López R, Muinelo Romay L (2016) Clinical Application of Circulating Tumour Cells in Prostate Cancer: From Bench to Bedside and Back. Int J Mol Sci 17: 1580. [Crossref]
- Miller MC, Doyle GV, Terstappen LW (2010) Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol 2010: 617421. [Crossref]
- Olmos D, Arkenau HT, Ang JE, Ledaki I, Attard G, et al. (2009) Circulating tumor cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single center experience. Ann Oncol 20: 27-33. [Crossref]
- Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, et al. (2007) Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13: 7053-7058. [Crossref]
- Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, et al. (2009) Circulating tumor cells as prognostic markers in progressive, castration-resistant prostate cancer: a re-analysis of IMMC38 trial data. Lancet Oncol 10: 233-239. [Crossref]
- Goldkorn A, Ely B, Quinn DI, Tangen CM, Fink LM, et al. (2014) Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol 32: 1136-1142. [Crossref]
- Danila DC, Anand A, Sung CC, Heller G, Leversha MA, et al. (2011) TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol 60: 897-904. [Crossref]
- Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, et al. (2009) Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res 69: 2912-8. [Crossref]
- Resel FL, Manso SJL, Galante RI, Moreno Sierra J, Olivier Gómez C (2012) Prognostic significance of circulating tumor cell count in patients with metastatic hormone-sensitive prostate cancer. Urology 80: 1328-1332. [Crossref]
- Scher HI, Heller G, Molina A, Attard G, Danila DC, et al. (2015) Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol 33: 1348-1355. [Crossref]
- Thalgott M, Heck MM, Eiber M, Souvatzoglou M, Hatzichristodoulou G, et al. (2015) Circulating tumor cells versus objective response assessment predicting survival in metastatic castration-resistant prostate cancer patients treated with docetaxel chemotherapy. J Cancer Res Clin Oncol 141: 1457-1464. [Crossref]
- Bitting RL, Healy P, Halabi S, George DJ, Goodin M, et al. (2015) Clinical phenotypes associated with circulating tumor cell enumeration in metastatic castration-resistant prostate cancer. Urol Oncol 33: 110.e1-9. [Crossref]
- Buskens CJ, Groot-Koerkamp B, Bemelman WA, Punt CJA (2012) Role of circulating tumor cells in metastatic colorectal cancer: clinical challenges and opportunities. Curr Colorectal Cancer Rep 8: 186-191.
- Wang JS, Foote MB, Jazieh KA, Diaz Jr LA (2013) The clinical potential of circulating tumor cells and circulating tumor-associated cellular elements in colorectal cancer. Curr Colorectal Cancer Rep 9: 303-311.
- Akagi Y, Kinugasa T, Adachi Y, Shirouzu K (2013) Prognostic significance of isolated tumor cells in patients with colorectal cancer in recent 10-year studies. Mol Clin Oncol 1: 582-592. [Crossref]
- Huang X, Gao P, Song Y, Sun J, Chen X, et al. (2015) Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 15: 202. [Crossref]
- Aggarwal C, Meropol NJ, Punt CJ, Iannotti N, Saidman BH, et al. (2013) Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol 24: 420-428. [Crossref]
- de Albuquerque A, Kubisch I, Stölzel U, Ernst D, Boese-Landgraf J, et al. (2012) Prognostic and predictive value of circulating tumor cell analysis in colorectal cancer patients. J Transl Med 10: 222. [Crossref]
- Groot KB, Rahbari NN, Buchler MW, Koch M, Weitz J (2013) Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a metaanalysis. Ann Surg Oncol 20: 2156-2165. [Crossref]
- Groot-Koerkamp B, Rahbari NN, Buchler MW, (2008) Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-II colorectal cancer after curative resection. Ann Surg Oncol 15: 2120-2128. [Crossref]
- Lu CY, Uen YH, Tsai HL, Chuang SC, Hou MF, et al. (2011) Molecular detection of persistent circulating tumor cells in stages II and III colon cancer patients via multiple blood sampling: prognostic significance of detection for early relapse. Br J Cancer 104: 1178-1184. [Crossref]
- Huang MY, Tsai HL, Huang JJ, Wang JY (2016) Clinical implications and future perspectives of circulating tumor cells and biomarkers in clinical outcomes of colorectal cancer. Translat Oncol 9: 340-347. [Crossref]
- Bidard FC, Proudhon C, Pierga JY (2016) Circulating tumor cells in breast cancer. Mol Oncol 10: 418-430. [Crossref]
- Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, et al. (2012) Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 13: 688-695. [Crossref]
- van Dalum G, van der Stam GJ, Tibbe AG, Franken B, Mastboom WJ, et al. (2015) Circulating tumor cells before and during follow-up after breast cancer surgery. Int J Oncol 46: 407-413. [Crossref]
- Rack B, Schindlbeck C, Jückstock J, Andergassen U, Hepp P, et al. (2014) Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 106: 5. [Crossref]
- Janni WJ, Rack B, Terstappen LW, Pierga JY, Taran FA, et al. (2016) Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res 22: 2583-2593. [Crossref]
- Banys-Paluchowski M, Krawczyk N, Meier-Stiegen F, Fehm T (2016) Circulating tumor cells in breast cancer--current status and perspectives. Crit Rev Oncol Hematol 97: 22-29. [Crossref]
- Andree KC, van Dalum G, Terstappen LW (2016) Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol 10: 395-407. [Crossref]
- Hall C, Karhade M, Laubacher B, Anderson A, Kuerer H, et al. (2015) Circulating tumor cells after neoadjuvant chemotherapy in Stage I-III triple-negative breast cancer. Ann Surg Oncol 22 Suppl 3: S552-558. [Crossref]
- Xenidis N, Perakki M, Apostolaki S, Agelaki S, Kalbakis K, et al. (2013) Differential effect of adjuvant taxane-based and taxane-free chemotherapy regimens on the CK-19 mRNA-positive circulating tumour cells in patients with early breast cancer. Br J Cancer 108: 549-556. [Crossref]
- Saad F, Pantel K (2012) The current role of circulating tumor cells in the diagnosis and management of bone metastases in advanced prostate cancer. Future Oncol 8: 321-331. [Crossref]
- Doyen J, Alix-Panabières C, Hofman P, Parks SK, Chamorey E, et al. (2012) Circulating tumor cells in prostate cancer: a potential surrogate marker of survival. Crit Rev Oncol Hematol 81: 241-256. [Crossref]
- Lowes LE, Lock M, Rodrigues G, D'Souza D, Bauman G, et al. (2012) Circulating tumour cells in prostate cancer patients receiving salvage radiotherapy. Clin Transl Oncol 14: 150-156. [Crossref]
- Scher HI, Morris MJ, Larson S, Heller G (2013) Validation and clinical utility of prostate cancer biomarkers. Nat Rev Clin Oncol 10: 225-234. [Crossref]
- Okegawa T, Itaya N, Hara H, Tambo M, Nutahara K. (2014) Circulating tumor cells as a biomarker predictive of sensitivity to docetaxel chemotherapy in patients with castration-resistant prostate cancer. Anticancer Res 34: 6705-6710. [Crossref]
- Li J, Gregory SG, Garcia-Blanco MA, Armstrong AJ (2015) Using circulating tumor cells to inform on prostate cancer biology and clinical utility. Crit Rev Clin Lab Sci 52: 191-210. [Crossref]
- Onstenk W, de Klaver W, de Wit R, Lolkema M, Foekens J, et al. (2016) The use of circulating tumor cells in guiding treatment decisions for patients with metastatic castration-resistant prostate cancer. Cancer Treat Rev 46: 42-50. [Crossref]
- Rahbari NN, Bork U, Kircher A, Nimitz T, Schölch S, et al. (2012) Compartmental differences of circulating tumor cells in colorectal cancer. Ann Surg Oncol 19: 2195-2202. [Crossref]
- Kawahara H, Watanabe K, Toyama Y, Yanagisawa S, Kobayashi S, et al. (2012) Determination of circulating tumor cells for prediction of recurrent colorectal cancer progression. Hepatogastroenterology 59: 2115-2118. [Crossref]
- Gazzaniga P, Gianni W, Raimondi C, Gradilone A, Lo Russo G, et al. (2013) Circulating tumor cells in high-risk nonmetastatic colorectal cancer. Tumour Biol 34: 2507-2509. [Crossref]
- Lu CY, Tsai HL, Uen YH, Hu HM, Chen CW, et al. (2013) Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. Br J Cancer 108: 791-797. [Crossref]
- Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, et al. (2013) Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol 131: 352-356. [Crossref]
- Zeng L, Liang X, Liu Q, Yang Z (2015) The Predictive Value of Circulating Tumor Cells in Ovarian Cancer: A Meta-Analysis. Int J Gynecol Cancer [Crossref]
- Kolostova K, Matkowski R, Jędryka M, Soter K, Cegan M, et al. (2015) The added value of circulating tumor cells examination in ovarian cancer staging. Am J Cancer Res 5: 3363-3375. [Crossref]
- Zhang Y, Li J, Cao L, Xu W, Yin Z (2012) Circulating tumor cells in hepatocellular carcinoma: detection techniques, clinical implications, and future perspectives. Semin Oncol 39: 449-460. [Crossref]
- Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, et al. (2013) Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer 133: 2165-2171. [Crossref]
- Sun YF, Xu Y, Yang XR, Guo W, Zhang X, et al. (2013) Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology 57: 1458-1468. [Crossref]
- Zhou J, Huang A, Yang XR (2016) Liquid Biopsy and its Potential for Management of Hepatocellular Carcinoma. J Gastrointest Cancer 47: 157-167. [Crossref]
- Tjensvoll K, Nordgård O, Smaaland R (2014) Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer 134: 1-8. [Crossref]
- Bidard FC, Huguet F, Louvet C, Mineur L, Bouché O, et al. (2013) Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol 24: 2057-2061. [Crossref]
- Han L, Chen W, Zhao Q (2014) Prognostic value of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Tumour Biol 35: 2473-2480. [Crossref]
- Riva F, Dronov OI, Khomenko DI, Huguet F, Louvet C, et al. (2016) Clinical applications of circulating tumor DNA and circulating tumor cells in pancreatic cancer. Mol Oncol 10: 481-493. [Crossref]
- Chang MC, Chang YT, Chen JY, Jeng YM, Yang CY, et al. (2016) Clinical significance of circulating tumor microemboli as a prognostic marker in patients with pancreatic ductal adenocarcinoma. Clin Chem 62: 505-513. [Crossref]
- Chapman CG, Waxman I (2016) Portal-vein blood samples as a new diagnostic entity for pancreatic cancer. Expert Rev Gastroenterol Hepatol 10: 665-667. [Crossref]
- Tien YW, Kuo HC, Ho BI, Chang MC, Chang YT, et al. (2016) A high circulating tumor cell count in portal vein predicts liver metastasis from periampullary or pancreatic cancer: a high portal venous CTC count predicts liver metastasis. Medicine (Baltimore) 95: e3407. [Crossref]
- Buglione M, Grisanti S, Almici C, Mangoni M, Polli C, et al. (2012) Circulating tumour cells in locally advanced head and neck cancer: preliminary report about their possible role in predicting response to non-surgical treatment and survival. Eur J Cancer 48: 3019-3026. [Crossref]
- Kulasinghe A, Perry C, Jovanovic L, Nelson C, Punyadeera C (2015) Circulating tumour cells in metastatic head and neck cancers. Int J Cancer 136: 2515-2523. [Crossref]
- Wu XL, Tu Q, Gilbert Faure, Patrice Gallet, Chantal Kohler, et al. (2016) Diagnostic and Prognostic Value of Circulating Tumor Cells in Head and Neck Squamous Cell Carcinoma: a systematic review and meta-analysis. Sci Rep 6: 20210. [Crossref]
- Si Y, Lan G, Deng Z, Wang Y, Lu Y, et al. (2016) Distribution and clinical significance of circulating tumor cells in nasopharyngeal carcinoma. Jpn J Clin Oncol 46: 622-630. [Crossref]
- Klinac D, Gray ES, Millward M, Ziman M (2013) Advances in personalized targeted treatment of metastatic melanoma and non-invasive tumor monitoring. Front Oncol 3: 54. [Crossref]
- Hoshimoto S, Faries MB, Morton DL, Shingai T, Kuo C, et al. (2012) Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma. Ann Surg 255: 357-362. [Crossref]
- Huang SK, Hoon DS (2016) Liquid biopsy utility for the surveillance of cutaneous malignant melanoma patients. Mol Oncol 10: 450-463. [Crossref]
- Qiao GL, Qi WX, Jiang WH, Chen Y, Ma LJ (2016) Prognostic significance of circulating tumor cells in esophageal carcinoma: a meta-analysis. Onco Targets Ther 9: 1889-1897. [Crossref]
- Wang HY, Wei J, Zou ZY, Qian XP, Liu BR (2015) Circulating tumour cells predict survival in gastric cancer patients: a meta-analysis. Contemp Oncol (Pozn) 19: 451-457. [Crossref]
- Inoue M, Otsuka K, Shibata H (2016) Circulating tumor cell count as a biomarker of a specific gastric cancer subgroup characterized by bone metastasis and/or disseminated intravascular coagulation - an early indicator of chemotherapeutic response. Oncol Lett 11: 1294-1298. [Crossref]
- Li Q, Zhi X, Zhou J, Tao R, Zhang J, et al. (2016) Circulating tumor cells as a prognostic and predictive marker in gastrointestinal stromal tumors: a prospective study. Oncotarget 7: 36645-36654. [Crossref]
- Zhang Z, Ramnath N, Nagrath S (2015) Current Status of CTCs as Liquid Biopsy in Lung Cancer and Future Directions. Front Oncol 5: 209. [Crossref]
- Truini A, Alama A, Dal Bello MG, Coco S, Vanni I, et al. (2014) Clinical Applications of Circulating Tumor Cells in Lung Cancer Patients by CellSearch System. Front Oncol 4: 242. [Crossref]
- Hou JM, Krebs MG, Lancashire L, Sloane R, Backen A, et al. (2012) Clinical significance, and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 30: 525-532. [Crossref]
- Hamilton G, Rath B, Holzer S, Hochmair M (2016) Second-line therapy for small cell lung cancer: exploring the potential role of circulating tumor cells. Transl Lung Cancer Res 5: 71-77. [Crossref]
- Wan JW, Gao MZ, Hu RJ, Huang HY, Wei YY, et al. (2015) A preliminary study on the relationship between circulating tumor cells count and clinical features in patients with non-small cell lung cancer. Ann Transl Med 3: 252. [Crossref]
- Bayarri-Lara C, Ortega FG, de Guevara ACL, Puche JL, Ruiz Zafra J, et al. (2016) Circulating tumor cells identify early recurrence in patients with non-small cell lung cancer undergoing radical resection. PLoS One 11: e0148659. [Crossref]
- Dorsey JF, Kao GD, MacArthur KM, Ju M, Steinmetz D, et al. (2015) Tracking viable circulating tumor cells (CTCs) in the peripheral blood of non-small cell lung cancer (NSCLC) patients undergoing definitive radiation therapy: pilot study results. Cancer 121: 139-149. [Crossref]
- Gazzaniga P, Gradilone A, de Berardinis E, Busetto GM, Raimondi C, et al. (2012) Prognostic value of circulating tumor cells in nonmuscle invasive bladder cancer: a CellSearch analysis. Ann Oncol 23: 2352-2356. [Crossref]
- Adamczyk LA, Williams H, Frankow A, Ellis HP, Haynes HR, et al. (2015) Current understanding of circulating tumor cells-potential value in malignancies of the central nervous system. Front Neurol 6: 174. [Crossref]
- Matthew EM, Zhou L, Yang Z, Dicker DT, Holder SL, et al. (2016) A multiplexed marker-based algorithm for diagnosis of carcinoma of unknown primary using circulating tumor cells. Oncotarget 7: 3662-3676. [Crossref]
- Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, et al. (2009) Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 15: 6980-6986. [Crossref]
- Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, et al. (2009) Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 175: 808-816. [Crossref]
- Krebs MG, Sloane R, Priest L, Lancashire L, Hou JM, et al. (2011) Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 29: 1556-1563. [Crossref]
- Punnoose EA, Atwal S, Liu W, Raja R, Fine BM, et al. (2012) Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 18: 2391-2401. [Crossref]
- O'Flaherty JD, Gray S, Richard D, Fennell D, O'Leary JJ, et al. (2012) Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer 76: 19-25. [Crossref]
- Tognela A, Spring KJ, Becker T, Caixeiro NJ, Bray VJ, et al. (2015) Predictive and prognostic value of circulating tumor cell detection in lung cancer: a clinician's perspective. Crit Rev Oncol Hematol 93: 90-102. [Crossref]
- Small AC, Gong Y, Oh WK, Hall SJ, van Rijn CJ, et al. (2012) The emerging role of circulating tumor cell detection in genitourinary cancer. J Urol 188: 21-26. [Crossref]
- Meye A, Bilkenroth U, Schmidt U, Füssel S, Robel K, et al. (2002) Isolation and enrichment of urologic tumor cells in blood samples by a semi-automated CD45 depletion autoMACS protocol. Int J Oncol 21: 521-530. [Crossref]
- Flaig TW, Wilson S, van Bokhoven A, Varella-Garcia M, Wolfe P, et al. (2011) Detection of circulating tumor cells in metastatic and clinically localized urothelial carcinoma. Urology 78: 863-867. [Crossref]
- Bluemke K, Bilkenroth U, Meye A, Fuessel S, Lautenschlaeger C, et al. (2009) Detection of circulating tumor cells in peripheral blood of patients with renal cell carcinoma correlates with prognosis. Cancer Epidemiol Biomarkers Prev 18: 2190-2194. [Crossref]
- Diamandis EP, Pantel K, Scher HI, Terstappen L, Lianidou E (2011) Circulating cancer cells and their clinical applications. Clin Chem 57: 1478-1484. [Crossref]


