Abstract
Objective
To determine the incidence and clinical presentations of preterm birth (PTB) <34 weeks gestation at the National Maternity Hospital (NMH), Dublin, Ireland and the University of Colorado Hospital (UCH), Denver, Colorado, between January 2007 to June 2008.
Study Design
Retrospective cohort study using data from perinatal databases. Pregnancies complicated by anomalies, multiple gestations, and fetal demise were excluded. PTB was categorized as resulting from preterm labor (PTL), premature rupture of the membranes (PROM), and medically indicated preterm birth (MIPTB). Data were analyzed using univariate analyses, c2, and Fisher exact test.
Results
There were 12 739 births at NMH and 4 029 at UCH with a total of 407 PTBs <34 weeks at NMH (1.7%) and UCH (4.8%), respectively (P<.0001). Spontaneous preterm labor, PPROM, and MIPTB occurred 4, 2, and 3 times more frequently at UCH versus NMH. Chorioamnionitis was 3-fold higher at UCH (13.2% vs. 3.2% at NMH, p=0.001).
Conclusion
Although the rate of PTB <34 weeks was significantly higher at UCH, the distribution of indications was remarkably similar. The incidence of chorioamnionitis was significantly higher at UCH. Further investigation of the similarities and differences between these populations is imperative in the effort to understand PTB and reduce its incidence worldwide.
Key words
preterm birth, prematurity, chorioamnionitis
Introduction
Preterm birth (<37 weeks gestation, PTB) is a major obstetric challenge accounting for up to three quarters of all perinatal mortality and over half of the long-term neurological morbidity identified in infants. Yet, the etiology of PTB remains elusive in most cases while the incidence continues to rise in many countries [1,2]. In 2008, PTB accounted for 12.3% of live births in the United States [3].While this rate has declined slightly in recent years, this is primarily on account of a reduction in late PTBs, occurring after 34 weeks gestation, in singleton pregnancies [3,4]. Increased multiple pregnancy rates associated with assisted reproduction and increased maternal age are also contributors to PTB [5].
Preterm birth is a public health concern with both short and long-term complications [4]. While even late PTB results in significant neonatal morbidity, the consequences of PTB occurring prior to 34 weeks are more severe, accounting for a significantly increased risk of mental retardation, vision impairment, and cerebral palsy [6]. Even in the absence of mortality and neurological morbidity, the adverse sequelae of PTB extend far beyond the neonatal period, reflected in an increased risk of cardiovascular disease, stroke, hypertension, and diabetes in adulthood. Additional long-term sequelae of prematurity continue to be identified each year [5] . To date, there are few effective interventions to prevent PTB, especially in first pregnancy. Thus, the imperative to elucidate the mechanisms of PTB so that effective prevention can be applied remains.
Considerable variability in PTB exists across national and international regions, with Africa and Asia having the highest rates of PTB in the world [1]. Europe has an overall lower rate of preterm birth (6.2%) when compared with the U.S (12.3%) [7-9]. Epidemiologic studies have consistently identified a variation in the incidence of PTB according to maternal ethnicity. In the United States, the rate of PTB among black women is 1.5 times higher than among white women with the incidence of recurrent PTB being four times higher [10]. These differences in PTB rates prompted a collaboration between researchers in Denver, Colorado and Dublin, Ireland. In contrast to the 11-12% incidence of PTB in the U.S the rate in Ireland is around 7% [1,4].
We chose to focus on births occurring prior to 34 weeks gestation as these account for the most severe neonatal complications. The aim of this study was to determine the incidence and clinical presentations of preterm birth (PTB) less than 34 weeks gestation among women who delivered at the National Maternity Hospital (NMH) Dublin, Ireland and the University of Colorado Hospital (UCH) Denver, Colorado, two tertiary care obstetric referral centers.
Materials/subjects and methods
This retrospective cohort study resulted from an ongoing collaboration and availability of perinatal databases at both the National Maternity Hospital (NMH) in Dublin, Ireland and the Department of Obstetrics and Gynecology at the University of Colorado Denver (UCH). Approval was obtained from the Institutional Review Boards of both institutions. We identified deliveries that occurred over a period of time when the institutions had very similar data repositories (January 2007 through June 2008) and included all deliveries after a gestational age of 23 weeks 6 days to 33 weeks 6 days from the perinatal databases of the National Maternity Hospital (NMH) in Dublin, Ireland, and the Department of Obstetrics and Gynecology at the University of Colorado Denver (UCH), Colorado, USA. The datasets were merged to incorporate select maternal risk factors and pregnancy outcomes. Because we were interested in the clinical presentation of singleton liveborn infants without anomalies delivered during the study period, multiple gestations and anomalous fetuses were excluded. Prior to data collection, definitions of all variables were standardized between the two institutions. Before analysis of the final dataset there was a secondary review of all records (by CH and RM) of PTB to assure that there was no misclassification of outcomes and to reconfirm the accuracy of gestational age at delivery.
Definition of outcomes
The primary outcome measures were the overall incidence of PTB before 34 weeks at the two institutions as well as the clinical presentation for PTB. Gestational age at delivery was defined on the basis of last menstrual period, earliest ultrasound, or the best estimate from the two. The incidence of preterm birth was calculated as the number of preterm births divided by the number of live, singleton births at the site during the time period. Preterm birth before 34 weeks was categorized as a result of spontaneous preterm labor (PTL), preterm premature rupture of the membranes (PPROM), or medically indicated PTB (MIPTB). Patients categorized as having PTL were diagnosed using the standard definition of painful contractions that affected cervical change and resulted in delivery. Patients with PPROM were diagnosed when they presented with rupture of the membranes greater than 24 hours before the onset of labor at both hospitals. PPROM patients were managed with a week of antibiotics to prolong latency at both institutions. We reviewed charts for evidence of PTL or PPROM complications including chorioamnionitis and placental abruption.
Chorioamnionitis was defined on the basis of usual clinical criteria including: maternal fever (temperature ≥38°C), maternal tachycardia, fetal tachycardia, and/or uterine tenderness [11]. Placental abruption was assigned to deliveries prompted by vaginal bleeding, usually with painful contractions in the absence of abnormal placentation or other etiology.
Secondary outcomes were mode of delivery and reason for MIPTB. Medically indicated preterm births were defined as those indicated due to a diagnosis of preeclampsia/eclampsia, abnormal placentation, maternal medical disease, intrauterine growth restriction (IUGR), isoimmunization/hydrops, or fetal heart rate tracing abnormalities necessitating delivery. Preeclampsia/eclampsia was defined using the 2000 “Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy” guidelines [12]. Assignment to the abnormal placentation group occurred if there was ultrasound evidence of a placenta previa, accreta, or percreta and vaginal bleeding prompting urgent delivery. Maternal medical diseases included any severe maternal condition for which delivery would improve overall maternal status. We defined IUGR as estimated fetal weight by ultrasound exam of <10th percentile, based on growth curves used at each institution, accompanied by Doppler or fetal heart rate tracing abnormalities necessitating delivery. Fetuses with antibody isoimmunization or other causes of fetal hydrops were delivered preterm if abnormal fetal status was documented. Abnormal fetal heart rate was defined as a biophysical profile of ≤4 of 8 or absent variability with repetitive variable or late decelerations on continuous fetal heart rate tracing. Maternal risk factors included in the analysis were maternal age, parity, race/ethnicity, history of preterm birth, tobacco use, tocolysis and transport status.
Statistical analysis
Data were analyzed in SAS 9.1 (SAS Institute, Cary, NC). Univariate analyses were used to generate descriptive statistics for the cohort. Associations were tested using the c2 or Fisher exact test. A p-value of <0.05 was considered significant.
Results
There were a total of 16 768 deliveries (12 739 at NMH and 4 029 at UCH) during the study period. There were 420 deliveries between 23 weeks 6 days and 33 weeks 6 days after excluding multiple gestations, fetal anomalies and IUFD. Complete information was available for 407 of these women (NMH n=212, UCH n=195).
We compare maternal risk factors for PTB and general outcomes between the two hospitals in Table 1. Patients at NMH were significantly older and predominantly white non-Hispanic. No patients in Dublin received tocolysis while 35% of patients in Denver received at least one type of tocolytic. At UCH, there were a higher percentage of transported patients than at NMH.
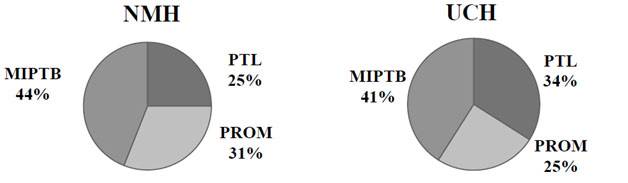
The incidence of PTB prior to 34 weeks gestation at NMH was 1.7% compared to 4.8% at UCH (P <.001) but the distribution of clinical indications for PTB was similar between hospitals. There were no statistical differences in cases of PTB <34 weeks due to PTL, PROM and MIPTB between NMH and UCH, Figure 1.
Differences in the reasons for a MIPTB between UCH and NMH
Differences in the reasons for MIPTB are presented in Table 2. The two centers did not differ in deliveries occurring secondary to preeclampsia, placenta previa, IUGR, isoimmunization/hydrops, or abnormal fetal heart rate. The only significant difference was the diagnosis of placental abruption in cases of MIPTB (15% at NMH vs. 4.8% at UCH, P=0.05).
We found a much higher incidence of chorioamnionitis at UCH, (13.2% versus 3.2% at NMH), table 3. We explored this further by looking at the rates stratified by race/ethnicity as well as the clinical presentation of PTB. There were significant differences in the incidence of chorioamnionitis when analyzed by race/ethnicity. Less than 3% of non-Hispanic white women in Ireland had diagnosed clinical chorioamnionitis versus 16.2% in the US. Chorioamnionitis was diagnosed in 7.7% of Irish patients with PROM versus 22.4% of American patients.
Discussion
Preterm birth rates before 34 weeks were almost 3 times higher in the Denver compared with the Dublin cohort. However, despite the differences in overall rates of PTB, the distribution of clinical presentations of PTB was similar when assessing rates of PTL, PPROM, and MIPTB.
Perhaps the most striking difference between the groups was the variation in ethnicity. Racial heterogeneity in the UCH, and homogeneity in the NMH, population appeared to heavily influence differences in PTB rates between the two countries. Forty-seven percent of the preterm births at <34 weeks occurred in Hispanic women followed by non-Hispanic white (34%) and black (11%) women at UCH. Conversely, white women comprised 85% of the PTB <34 weeks at NMH with 5% black women and smaller percentages from other groups. Within the US, there is a strong correlation between socioeconomic status, race, and preterm birth [5,13]. Well-documented disparities in the risk of spontaneous PTB and PPROM among different racial/ethnic groups exist [3]. For example, when compared with non-Hispanic white infants, non-Hispanic black infants are more than 1.5 times as likely to be born preterm [1,14-16]. In Colorado, non-Hispanic white individuals comprise 71.0% of the population followed by 20.0% Hispanic and 4.3% non-Hispanic black. Colorado’s racial/ethnic composition differs somewhat from the overall US population of 65.6% non-Hispanic white, 15.4% Hispanic, and 12.8% non-Hispanic black individuals, yet our population still exhibits racial disparities in birth outcomes [3,17]. Patel et al. [18] found the risk of PTB in the United Kingdom to be higher in black and Asian women than in white European women, however the discrepancy was smaller than that seen in US women.
Chorioamnionitis was diagnosed more than twice as frequently at UCH versus NMH in both the PTL and PROM groups. One explanation is that, at UCH, there was a higher incidence of chorioamnionitis secondary to a higher proportion of Hispanic and non-Hispanic black women. Risk factors for chorioamnionitis are similar to those for PTL and are impacted by maternal race and ethnicity with rates being higher among African-American women [5,19-22]. Differences in the incidence of chorioamnionitis between the two sites may be secondary to racial/ethnic differences between the two hospitals. It may also be partially explained by newer research on racial and ethnic differences in inflammatory milieu and microbiomes [23-25]. However, we also saw a higher rate of chorioamnionitis in non-Hispanic white women at UCH. Therefore, we examined other explanations. One alternate explanation may be the use of tocolytics commonly, during the study period, at UCH whereas tocolysis was, and is, not used at NMH. This difference in practice may have impacted overall incidences of chorioamnionitis between the two institutions as preterm birth is known to be associated with intraamniotic inflammation and infection [26,27]. It is possible that tocolysis use may have attenuated preterm delivery until chorioamnionitis was clinically evident at UCH, as the majority of the UCH cases of tocolysis use and chorioamnionitis occurred in women with PTL, not PPROM. The few cases of chorioamnionitis that occurred at NMH were predominantly in the PPROM group. Perhaps the cases of PTL occurring at NMH were less likely to be complicated by chorioamnionitis because tocolytics were not used and, we speculate, these patients were delivered prior to the overt diagnosis of chorioamnionitis. In any case, the use of tocolysis did not positively impact overall PTB rates at <34 weeks gestation. Larger numbers are needed to better answer these questions.
National maternity hospital and UCH patients had similar rates of medically indicated preterm births at <34 weeks for the most common indications. However rates of placental abruption differed significantly between the two groups. Despite our attempts at standardizing definitions of each outcome, it is possible that there was a discrepancy in classification of placental abruption between the two centers.
Limitations of this study include a lack of information on placental pathology in a large number of cases. This information may have elucidated the differences in chorioamnionitis and placental abruption between the two centers. Additionally, controlling for nuances in management, diagnosis, and clinical judgment amongst various providers was not technically feasible. Patients presenting with preterm labor in the U.S. and Ireland are managed differently with respect to specific antibiotics, timing of antenatal corticosteroids, tocolysis and other practices.
Morken et al.[28] compared three Scandinavian countries and trends in preterm deliveries and found an increase in preterm births of less than or equal to a 1% increase over 10 years [28]. Comparing regions and countries, however, is difficult as management, resuscitation, and reporting practices differ [8]. As noted in a study of mortality patterns among very preterm babies in France and the UK, comparison was difficult due to differences in international interpretation of perinatal and infant data. There are no published investigations of preterm pregnancy management and outcomes between the U.S. and Ireland.
Our study provides a contemporaneous assessment of notable differences that exist in the incidence and clinical presentations of preterm births <34 weeks gestation between 2 university hospitals in developed western countries. Investigation of the similarities and differences between these populations provides some insight into the pathogenesis of PTB and to the translational research ongoing in this field. This study underscores the dire need for further investigation into the socioeconomic and cultural differences that may impact this process and how these differences may be applied towards an effective preventive clinical intervention.
This article is being submitted with the full knowledge and approval of the University of Colorado Hospital, Denver, Colorado, USA, the National Maternity Hospital, Dublin, Ireland, and all authors. The authors declare no conflict of interest.
References
- March of Dimes White Paper on preterm birth: The global and regional toll.
- Burke C, Morrison JJ (2000) Perinatal factors and preterm delivery in an Irish obstetric population. J Perinat Med 28: 49-53. [Crossref]
- (2015) MOD vital statistics. marchofdimes.org/Peristats.
- McIntire DD, Leveno KJ (2008) Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol 111: 35-41. [Crossref]
- Spong CY (2009) Preterm birth: an enigma and a priority. Obstet Gynecol 113: 770-771. [Crossref]
- Rich-Edwards JW, Stampfer MJ, Manson JE, Rosner B, Hankinson SE, et al. (1997) Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 315: 396-400. [Crossref]
- Savitz DA (2008) Invited commentary: disaggregating preterm birth to determine etiology. Am J Epidemiol 168: 990-992, discussion 993-994. [Crossref]
- Berkowitz GS, Blackmore-Prince C, Lapinski RH, Savitz DA (1998) Risk factors for preterm birth subtypes. Epidemiology 9: 279-285. [Crossref]
- Ananth CV, Vintzileos AM (2006) Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med 19: 773-782. [Crossref]
- Kistka ZA, Palomar L, Lee KA, Boslaugh SE, Wangler MF, et al. (2007) Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol 196: 131.e1-6. [Crossref]
- Newton ER, Prihoda TJ, Gibbs RS (1989) Logistic regression analysis of risk factors for intra-amniotic infection. Obstet Gynecol 73: 571-575. [Crossref]
- (2000) Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 183: S1-S22. [Crossref]
- Orr ST, James SA, Reiter JP (2008) Unintended pregnancy and prenatal behaviors among urban, black women in Baltimore, Maryland: the Baltimore preterm birth study. Ann Epidemiol 18: 545-551. [Crossref]
- Macdorman MF, Mathews TJ (2008) Recent trends in infant mortality in the United States. NCHS Data Brief 1-8. [Crossref]
- Anachebe NF, Sutton MY (2003) Racial disparities in reproductive health outcomes. Am J Obstet Gynecol 188: S37-42. [Crossref]
- Ahern J, Pickett KE, Selvin S, Abrams B (2003) Preterm birth among African American and white women: a multilevel analysis of socioeconomic characteristics and cigarette smoking. J Epidemiol Community Health 57: 606-611. [Crossref]
- US census data. 2008.
- Patel RR, Steer P, Doyle P, Little MP, Elliott P (2004) Does gestation vary by ethnic group? A London-based study of over 122,000 pregnancies with spontaneous onset of labour. Int J Epidemiol 33: 107-113. [Crossref]
- Harger JH, Hsing AW, Tuomala RE, Gibbs RS, Mead PB, et al. (1990) Risk factors for preterm premature rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol 163: 130-137. [Crossref]
- Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371: 75-84. [Crossref]
- Zhang J, Savitz DA (1992) Preterm birth subtypes among blacks and whites. Epidemiology 3: 428-433. [Crossref]
- Holzman C, Lin X, Senagore P, Chung H (2007) Histologic chorioamnionitis and preterm delivery. Am J Epidemiol 166: 786-794. [Crossref]
- Manuck TA, Esplin MS, Biggio J, Bukowski R, Parry S, et al. (2015) The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol 212: 487.e1-487.e11. [Crossref]
- Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, et al. (2014) Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci 21: 32-40. [Crossref]
- Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, et al. (2014) Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 160: 2272-2282. [Crossref]
- Menon R, Taylor RN, Fortunato SJ (2010) Chorioamnionitis--a complex pathophysiologic syndrome. Placenta 31: 113-120. [Crossref]
- Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, et al. (1989) Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 161: 817-824. [Crossref]
- Morken NH, Vogel I, Kallen K, Skjaerven R, Langhoff-Roos J, et al. (2008) Reference population for international comparisons and time trend surveillance of preterm delivery proportions in three countries. BMC Womens Health 8: 16.