The aim of this study was to evaluate how de and remineralization cycles with and without fluoride would affect the surface microhardness and surface roughness of dental enamel. Fifty seven polished human enamel slabs were divided into 5 groups. The specimens of 4 experimental groups were exposed to one kind of demineralized and 2 kinds of remineralized solutions in 2 types of de and remineralization cycles. The 2 kinds of remineralized solutions were artificial saliva with and without fluoride. The specimens were immersed in 2 types of de and remineralization cycles for 4 times for 5 minutes, and 20 times for 1 minute, respectively. The control group was polished and refrigerated in saline solution. After being immersed in these solutions, Knoop surface microhardness (SMH) and surface arithmetic mean roughness (Ra) were measured. Statistical analysis of average SMH and Ra among 5 groups was carried out using 1-way ANOVA and Tuckey’s test. A 2-way ANOVA was used to analyze average SMH and Ra with de and mineralization cycles and fluoride addition to remineralization solution as factors. Except for the 5-minute-cycle group remineralized with fluoride, SMH of all other 3 experimental groups had significantly decreased as compared to the control group. The results of 2-way ANOVA for SMH showed that both 5- minute-cycle and those remineralized with fluoride were significantly higher than others. The results of 2-way ANOVA for Ra showed that remineralization solution with fluoride significantly induced larger enamel crystal. Thus, longer de and remineralization cycles increased SMH, remineralization with fluoride enhanced remineralization, and enamel crystal growth increased SMH and Ra. This study indicated that remineralized enamel crystal was different from crystal before it demineralized and that fluoride played an important role in preventing enamel erosion.
de and remineralization cycle, fluoride, erosion, surface micro hardness, surface average roughness
Dental erosion is known as the loss of tooth structure due to chemical process without the involvement of bacteria, triggered by extrinsic and intrinsic factors. Among all extrinsic factors, it is reported that acidic foodstuffs and drinks are major etiological causes [1]. Therefore, many papers have studied the behavior of enamel erosion with acidic drinks or solution in vitro. To better understand the mechanism of dental erosion, most of these studies have utilized highly controlled artificial conditions, such as long de or remineralization time to study individual risk factors. Many studies have used different immersion methods to reveal early signs of dental erosion. Lussi [2] immersed enamel blocks in acidic drinks and solutions for 20 minutes. Attein [3] let enamel samples to erode in beverage for 1, 5, and 15 minutes. In Eisenburger [4], the effect of remineralization time was studied after enamel specimens eroded for 2 hours in citric acid which were again remineralized for 1, 2, 4, 6, 9 and 24 hours respectively. Barac [5] studied enamel samples which were first exposed to soft drinks for 15, 30 and 60 minutes, and then left in filtered saliva until the next immersion for 3 times per day for 10 days. However, de and remineralization on enamel surface is a daily occurrence of cycles. Although saliva has been the most important biological factor in the prevention of dental erosion [6]; there are few in vitro studies which reproduce the process of de and remineralization in oral environment with saliva. While fluoride is thought to accelerate remineralization [7], few studies have reported conclusive results on the influence of CaF2-like, which is formed under high fluoride concentration, on the prevention of dental erosion [8,9].
As enamel erosive demineralization begins with a partial loss of enamel mineral, which causes an initial surface softening and roughness [10], measurements on surface hardness and surface roughness are often used to determine erosive alterations of dental hard tissues [11].
Thus, in this study, we aim to evaluate how de and remineralization cycles with and without high concentrated fluoride would affect Knoop surface microhardness and surface arithmetic mean roughness.
Preparation of enamel specimens
Eight carries-free human molars extracted for periodontal or medical reasons were used. Soft tissue debris was removed from the teeth and refrigerated in saline solution. After checking for damage on the surface, 57 specimens were cut (3 × 3 × 2 mm) from the teeth using a diamond disk and each specimens was embedded in acrylic resin (Scandiquck, Scandia, Germany). These specimens were polished by using polishing machine (Scandimatic universal 33035, Scandia, Germany) with #800, #1000, #1200, #1600, #2000 and #2400 emery paper disks and buff with 5.00, 0.30 and 0.05 μm Al2O3 suspensions (Guaranteed reagent, Refinetec, Japan). They were divided into 5 groups with same average surface microhardness. All specimens were refrigerated in saline solution when not used for experiments.
Preparation of De and remineralization solution
Demineralizing solution was prepared with acetic acid adjusted to pH 3.5 in distilled water.
Artificial saliva containing 0.7 mmol/l CaCl2・2H2O, 0.2 mmol/l MgCl2・6H2O, 4 mmol/l KH2PO4, 30 mmol/l KCl, 20 mmol/l Hepes, pH7.0 [4] was used as remineralizing solution. Remineralizing solution containing fluoride was prepared with the artificial saliva and NaF adjusted to 225 ppmF.
De and remineralization (Figure 1)
The specimens of experimental 4 groups were repeatedly immersed in 20ml demineralized solutions, 20ml distilled water and 20ml remineralized solution at 25 °C by using tissue processor (EM TP, Leica Microsystems, Japan). Group 1 (n=12) was exposed to demineralized and remineralized solutions 4 times for 5 minutes respectively. Group 2 (n=12) was exposed to demineralized and 225ppmF fluoridated remineralized solutions 4 times for 5 minutes respectively. Group 3 (n=12) was exposed to demineralized and remineralized solutions 20 times for 1 minute respectively. Group 4 (n=12) was exposed to demineralized and 225ppmF fluoridated remineralized solutions 20 times for 1 minute respectively. Group 5 (n=9) was refrigerated in saline solution as a control group of this study.
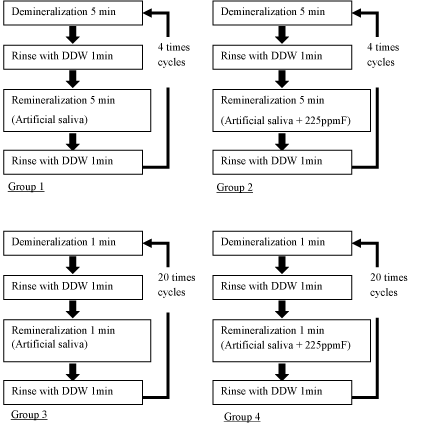
Figure 1. De and remineralization cycles of experimental 4 groups.
Measurements of microhardness and roughness
Before Knoop surface microhardness (SMH) measurements, the specimens were rinsed with distilled water for 10 seconds, wiped with absorbent paper and dried in air for 2 minutes. SMH measurements were performed with Knoop diamond which has a load of 50g and a dwell time of 15 seconds on a hardness tester (HM-101, Mitutoyo, Japan). Indentations on each specimen were made randomly, and the length of each indentation was measured with an optical analysis system. The indentation lengths were used for the calculation of the SMH value.
Arithmetic mean roughness (Ra) was measured by using Nano Hybrid Microscope (VN-8010, Keyence, Japan) with Si catilever (scan speed 0.9 μm/sec, measurement mode DFM-H, resolution pixels 512 × 512). Ra measurements were performed on 3 areas randomly in each specimens.
Statistical analysis of average SMH and Ra of all 5 groups was carried out using 1-way ANOVA and Tuckey’s test. In addition, 2-way ANOVA was used to analyze average SMH and Ra of experimental 4 groups, with de and mineralization cycles and remineralization solution with and without fluoride as factors. The statistical analysis were performed using the statistical software SPSS statistics (IBM SPSS Statistics version 23.0, IBM Japan, Japan) with p < 0.05 as a statistically significant value.
Results of SMH
One-way ANOVA results of SMH showed the significant difference in SMH among 5 groups (p < 0.001). Group 2 had the highest SMH among the experimental groups and there was no significant difference between group 2 and group 5 (control group) (Table 1). Except for group 2 (p < 0.001), SMH of other experimental groups (group 1, 3, 4) decreased significantly as compared to the control group. SMH of group 2 was significantly higher than group 3 (p < 0.01) and group 4 (p < 0.05). Two-way ANOVA results of SMH showed that both de and remineralization cycles and fluoride addition were significant factors (p < 0.05) (Table 2). SMH decreased significantly with 1-minute-cycle of de and remineralization than 5-minute-cycle (p < 0.05), remineralization with fluoride significantly inhibited the decrease of SMH than remineralization without fluoride (p < 0.05).
Table 1. The values of Knoop surface microhardness.
HK (Mean ± SD)
|
Artificial Saliva |
Artificial Saliva + 225 ppm F |
5-minutes-cycle |
Group 1 |
96.95 ± 33.65a |
Group 2 |
134.95 ± 37.10de |
1-minute-cycle |
Group 3 |
84.14 ± 20.37bd |
Group 4 |
96.44 ± 41.11ce |
Before exposure |
Group 5 |
169.10 ± 29.37abc |
Same letters indicate significant difference among the groups.
abc: p < 0.001, d: p <0 .01, e: p < 0.05
Table 2. Two-way ANOVA tables for Knoop surface microhardness.
Source |
Some of square |
Degree of freedom |
Mean square |
F value |
p value |
Contribution (%) |
Fluoride addition |
7589.7 |
1 |
7589.7 |
6.582 |
0.014 |
9.4 |
De and remineraization cycle |
7900.8 |
1 |
7900.8 |
6.851 |
0.012 |
9.9 |
Fluoride addition × De and remineraization cycle |
1980.3 |
1 |
1980.3 |
1.717 |
0.197 |
1.2 |
error |
50739.1 |
44 |
1153.2 |
|
|
79.5 |
Total |
68209.8 |
47 |
|
|
|
100.0 |
Results of Ra
Group 1 had the highest Ra and Group 4 had the lowest (Table 3). One-way ANOVA results of Ra showed that there is no significant difference among the 5 groups (p = 0.069). Tukey’s test results of Ra showed no significant difference between each group. Two-way ANOVA results of Ra indicated that fluoride addition to remineralization solution was the only significant factor (p<0.05) (Table 4). Ra decreased significantly when specimens were remineralized with fluoride than without fluoride (p < 0.05).
Table 3. The values of surface arithmetic mean roughness.
nm (Mean ± SD)
|
Artifical Saliva |
Artifical Saliva + 225 ppm F |
5-minutes-cycle |
Group 1 |
205.88 ± 46.62 |
Group 2 |
153.77 ± 71.91 |
1-minute-cycle |
Group 3 |
177.31 ± 63.80 |
Group 4 |
139.26 ± 56.87 |
Before exposure |
Group 5 |
144.12 ± 67.29 |
Table 4. Two-way ANOVA tables for surface arithmetic mean roughness.
Source |
Some of square |
Degree of freedom |
Mean square |
F value |
p value |
Contribution (%) |
Fluoride addition |
24384.1 |
1 |
24384.1 |
6.659 |
0.013 |
10.8 |
De and remineraization cycle |
5564.2 |
1 |
5564.2 |
1.519 |
0.224 |
1.0 |
Fluoride addition × De and remineraization cycle |
593.1 |
1 |
593.1 |
0.162 |
0.689 |
- |
error |
161124.9 |
44 |
3661.9 |
|
|
88.2 |
Total |
191666.3 |
47 |
|
|
|
100.0 |
Many studies have demonstrated that demineralization of enamel would results in a significant reduction in microhardness [2,3]. Knoop hardness values are most frequently used for brittle materials or thin sections due to their relatively superficial indentations [12]. Therefore, Knoop surface microhardness has been used to evaluate not only dental caries but also dental erosion. Furthermore, atomic force microscopy (AFM) and nanoindentation, which is used to measure surface roughness, have been used to study dental erosion [13,14]. To measure early erosion, we used surface microhardness measurement and surface roughness measurement in this study.
In this study, while the analysis of SMH in the present study affirmed, de and remineralization cycles were significant factors, 5-minute-cycle was most significant in inhibiting dental erosion. Despite of the difference in cycles, group 1 and 2 with 4 cycles and group 3 and 4 with 20 cycles, the specimens had similar length of immersion in de and remineralization solution. Dissolution is a process when ions separate from crystal. In very dilute solution, the activity of an ion is similar to its concentration, but as the soluble salt concentration increases, the activity becomes significantly less than the concentration because of ion interractions [15]. Therefore, the activity of ions and reaction of dissolution of a 5-minute demineralization cycle is considered lower than that of a 1-minute cycle in this study. Furthermore, contact with acidic solutions firstly leads to dissolution of the enamel prism sheath, then of the cores and finally of the interprismatic areas [3]. Our study also suggested that the structure of enamel crystal changes accordingly to the length of each cycle.
Newly precipitated crystal forms after remineralization, are usually small and contain many defects, e.g. missing ions in the lattice, which make the crystal more soluble [15]. Over time, these more soluble parts tend to re-form and the crystals grow to reach their maximum natural size [15]. In this study, new enamel crystal formed after 5-minute remineralization cycle was harder than that of a 1-minute cycle; suggesting that the enamel crystal might have grown larger and became more insoluble.
CaF2 is a compound formed when the fluoride concentrations in the solution bathing enamel are higher than 100 ppm, and acts as a pH-controlled fluoride and calcium reservoir [9]. The dissolution rate of CaF2 is limited by the adsorption of HPO42- that is lost under acidic pH, thus allowing CaF2 to dissolve and fluoride and calcium to be released [9]. Studies have demonstrated that applying fluoride formulations with the sole purpose of enhancing the formation CaF2-like deposits to prevent dental erosion will provide only a limited protective potential against erosion [8]. On the other hand, the presence of small quantities of fluoride solution in between the crystals is also likely to encourage precipitation of fluor (hydoroxy) apataite and hence remineralization [9]. With the addition of fluoride, the results suggested the formation of CaF2 and free F- by CaCl2・2H2O and NaF in remineralized solution. On top of that, free F- reduced the solubility of enamel and enhanced remineralization. Therefore, this study demonstrated a significant influence of fluoride in SMH and Ra through remineralization.
Average Ra measured by using profilometer [5,12] or atomic force microscopy [16] is a suitable evaluation for dental erosion at the microscale and nanoscale. Enamel immersed in 500 ppm NaF solution had an effect of reducing roughness significantly, and NaF has the ability to smooth the surface of enamel [16]. The results in this study also showed a reduced Ra in remineralized solution with fluoride; hence, an indication of the ability of NaF to improve the nanomechanical properties, which includes its ability to smoother the enamel surface.
However, the present study has two limitation. Firstly, only in vitro study was conducted. Secondly, polished teeth were used in an attempt to standardize specimens although it is noted that polished tooth surfaces showed more pronounced enamel dissolution than natural tooth [17]. Furthermore, this study could not reproduce a complete erosive process in a complex oral environment.
Despite the limitations, our study was able to demonstrate preventing enamel erosion with longer of de and remineralization cycles as a cause to decrease SMH and remineralization with fluoride as a cause decrease SMH and Ra. This study also indicated that remineralized enamel crystal was a different form of crystal before it demineralized and that fluoride played an important role in preventing enamel erosion.
This work was supported by JSPS KAKENHI 15K11442.
- Lussi A, Jaeggi T, Zero D (2004) The role of diet in the aetiology of dental erosion. Caries Res 38: 34-44. [Crossref]
- Lussi A, Jäggi T, Schärer S (1993) The influence of different factors on in vitro enamel erosion. Caries Res 27: 387-393. [Crossref]
- Attin T, Koidl U, Buchalla W, Schaller HG, Kielbassa AM, et al. (1997) Correlation of microhardness and wear in differently eroded bovine dental enamel. Arch Oral Biol 42: 243-250. [Crossref]
- Eisenburger M, Addy M, Hughes JA, Shellis RP (2001) Effect of time on the remineralisation of enamel by synthetic saliva after citric acid erosion. Caries Res 35: 211-215. [Crossref]
- Barac R, Gasic J, Trutic N, Sunaric S, Popovic J, Djekic P, et al. (2015) Erosive effect of different softdrinks on enamel surface in vitro: Application of stylus profilometry. Med Princ Pract 24: 451-457. [Crossref]
- Hara AT, Lussi A, Zero DT (2006) Biological factors. Monogr Oral Sci 20: 88-99. [Crossref]
- Li X, Wang J, Joiner A, Chang J (2014) The remineralisation of enamel: a review of the literature. J Dent 42: S12-20. [Crossref]
- Lussi A, Carvalho TS (2015) The future of fluorides and other protective agents in erosion prevention. Cares Res 49: 18-29. [Crossref]
- Buzalaf MA, Pessan JP, Honório HM, ten Cate JM (2011) Mechanisms of action of fluoride for caries control. Monogr Oral Sci 22: 97-114. [Crossref]
- Carvalho TS, Lussi A (2014) Combined effect of a fluoride-, stannous- and chitosan-containing toothpaste and stannous-containing rinse on the prevention of initial enamel erosion-abrasion. J Dent 42: 450-459. [Crossref]
- Attin T (2006) Methods for assessment of dental erosion. Monogr Oral Sci 20: 152-172. [Crossref]
- Field JC, German MJ, Waterhouse PJ (2014) Qualifying the lapped enamel surface: A profilometric, electron microscopic and microhardness study using human, bovine and ovine enamel. Arch Oral Biol 59: 455-460. [Crossref]
- Poggio C, Lombardini M, Dagna A, Chiesa M, Bianchi S (2009) Protective effect on enamel demineralization of a CPP-ACP paste: an AFM in vitro study. J Dent 37: 949-954. [Crossref]
- Poggio C, Ceci M, Beltrami R, Lombardini M, Colombo M (2014) Atomic force microscopy study of enamel remineralization. Ann Stomatol (Roma) 5: 98-102. [Crossref]
- ten Cate JM, Larsen MJ, Pearce EIF, Fejerskov O (2008) Chemical interactions between tooth and oral fluids. In Fejerskov O, Kidd E (ed), Dental Caries the disease and its clinical management. Second edition, Munksgaard: Blackwell.
- Zhou C, Zhang D, Bai Y, Li S (2014) Casein phosphopeptide-amorphous calcium phosphate remineralization of primary teeth early enamel lesions. J Dent 42: 21-29. [Crossref]
- Ganss C, Klimek J, Schwarz N (2000) A Comparative profilometric in vitro study of the susceptibily of polished and natural human enamel and dentine surface to erosive demineralization. Arch Oral Biol 45: 897-902. [Crossref]

