Abstract
Background and purpose: This study was undertaken to investigate the development changes of myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation expression in acute ischemia hippocampus and cortex, to establish the relationship between these changes and ischemia damage.
Methods: The acute multi-cerebral infarction model was established by method of Kaneko. The histopathological analyses was observed by microscope (HE staining). The MARCKS and p-MARCKS protein expression were surveyed by western blot technic and immunohistochemical test method.
Results: We found that in all model groups, hippocampus and cortex synchronous changed obvious in histopathology. The expression of the MARCKS and p-MARCKS protein phosphorylation was seen in neuron cytoplasm, cytomembrane. MARCKS and p-MARCKS protein in hippocampus and cortex were expressed in normal group vs. other groups (p<0.01). The changes of MARCKS and p-MARCKS protein expression are identical. After acute ischemic , the expression are all increased, the expression of 1h start heighten, the expression of 24h is supreme, form dynamic changes. The expression of 24h obviously super other group. We gave CTM rats dropping pilula at 24h after ischemia. The damage of CTM neuron have more reckless than the model group and the WM group in histopathology. And the MARCKS、p-MARCKS protein expression were lower than the model group.
Conclusions: MARCKS phophorylation, correlate with the occurring and extent neuron damage after acute cerebral ischemia. Signal transduction abnormality maybe the important mechanism of ischemia damage. Qingnao drop pilula could intervene PKC-MARCKS signal transduction system, to turn slower MARCKS phophorylation, and to delay the process of neuron death, can lighten acute cerebral ischemia damage.
Key words
acute multi-cerebral infarction, hippocampus, cortex, myristoylated alanine-rich C kinase substrate, phosphorylation
Introduction
Signal transduction pathway abnormality is a major pathological feature of acute multi-cerebral infarction, yet cellular mechanisms regulating secretion of signal transduction pathway have not been fully elucidated. Activation of downstream protein kinase C (PKC) pathway is evidenced by increase in the phosphorylation of a common PKC substrate-Myristoylated Alanine-rich C Kinase Substrate (MARCKS) [1]. If the phosphorylation of MARCKS was increased, the major presynapic substrate for PKC, this effect was also significantly attenuated by PKC inhibition [2].
MARCKS is a protein correlatived to cerebral growth and development , and reside in central nervous system mainly hippocampus and cortex. MARCKS, as a ubiquitously expressed substrate of protein kinase C (PKC) that is involved in reorganization of the actin cytoskeleton [3], consuming ATP, occurring phosphorylation, participating transmembrane information transfer and cell function regulative process. The liveness of PKC-MARCKS signal transduction pathway is concerned with the development and level of acute cerebral ischemia. The central domain of MARCKS is both basic (zeta=13) and hydrophobic (five Phe residues), and is flanked with two long chains, one ending with the myristoylated N-terminus. This natively unfolded protein is modeled as a flexible chain of "beads" representing the amino acid residues [4].
MARCKS cross-links filamentous actin (F-actin) and regulates its reorganization. This activity is reduced either by PKC-induced MARCKS phosphorylation (PKC pathway) or by its direct binding to calmodulin (CaM; CaM pathway), both inducing MARCKS translocation, F-actin reorganization, and exocytosis (CGE) [5]. The cell receive extracellular signal and introduce it to intra-cellular, to direct homologus genetic transcription and protein express, and to accommodate cellular biological function. This process is not much of accomplished by a signal transduction pathway. Frequently to demand many signal transduction pathways of information communication and function cooperation. MARCKS protein belong to “natural not yet fold protein”, can implement interaction function. To confirm that the development changes of MARCKS phosphorylation expression in acute ischemia hippocampus and cortex, to establish the relationship between these changes and ischemia damage.
Results
The appearance of acute multiple cerebral ischemia rats
Model rats are 85, only live 75. Survival rate is 88. 24%. 24h after ischemia, each group of rats appeared different nerve function defect symptoms. The normal group and sham control group rats could stand, gait flexible, filed their tails with hand, their limbs stretched out symmetrical; The symptom of model group varys in degree, light one appeared slow or reduce motion, irritability, serious one followed by circle or could not stand, the body dumped to a side, hemiplegia, convulsions or incontinence, etc. We filed a rat tail with hand, its forelimb buckling, hindlimb straight and its limbs stretched out asymmetric.
The scores of neurological function deficits in acute multiple cerebral ischemia rats
24h after brain ischemia, the scores of neurological function deficits of rats in 24h, 72h, 7d after ischemia group were 2.18, 2.12, 2.41 respectively (the average score was 2.24), indicating the obvious neurological function deficits.
Hippocampus and cortex histological change of acute multiple cerebral infarction rats
Nomal group: Neurocyte were regular alignment, and closely, neat. The nuclear membrane and nucleoli were clear. Sham control group:The number and arrangement of neurocyte were the same as nomal group. 1h after ischemia group:The structure of neurocyte was close to nomal, no obvious pathologic change. 6h after ischemia group: neurocyte arrenged sparsely, not neat, cells have reduced, depigmentation. 24h after ischemia group: Brain tissue was necrotic, edema, neurocyte arranged untidy, depigmentation, high swelling, cytoplasm shining brilliantly, nuclear pyknosis. 72h after ischemia group: Neurocyte arranged sparsely, untidy, the number decreased significantly, depigmentation phenomenon serious, irregular shape, nuclear pyknosis. 7d after ischemia group: Neurocyte arranged sparsely, number reduced significantly, cell shrinkage, nuclear pyknosis (Figure 1).
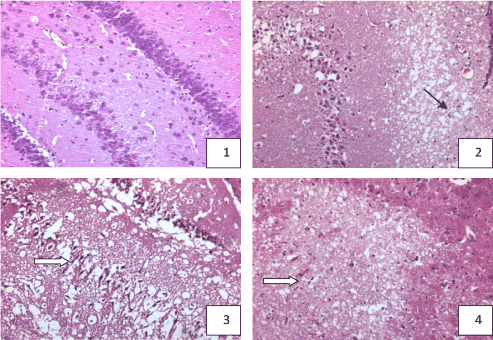
Figure 1. hippocampus and cortex pathologic change in the rat with acute multi-cerebral infarction (HE Staining × 100)
- Normal: neurocyte abundant, regular alignment , no pathologic change in hippocampus dentate nucleus and CA4 area
- 24h after ischemia: neurocyte reduced, survival neurocyte edema, karyopyknosis in hippocampus, a focus of cribriform infection
- 24h after ischemia: neurocyte reduced, survival neurocyte edema, karyopyknosis in hippocampus
- 7d after ischemia: focus of infarct in cortex
The MARCKS, p-MARCKS protein expression in rat hippocampus with acute multicerebral infarction
The MARCKS and p-MARCKS protein expression were surveyed by western blot technic. MARCKS and p-MARCKS protein were expressed in 1h after ischemia group (M1h), 6h after ischemia group (M6h), 24h after ischemia group (M24h), 72h after ischemia group (M72h), 7d after ischemia group (M7d) vs. Nomal group (CONT), Sham control group (SH) . The changes of MARCKS and p-MARCKS protein expression are identical. After acute ischemic, the expression are all increased, the expression of 1h start heighten, the expression of 24h is supreme, form dynamic changes. The expression of 24h obviously super other group (p<0. 01) (Figure 2).
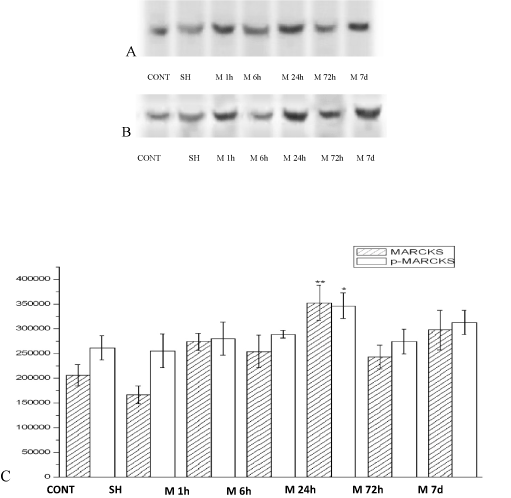
Figure 2. The MARCKS (A)、p-MARCKS (B) expression in rat hippocampus with acute multicerebral infarction (C). ( ± s, n=6)
The MARCKS、p-MARCKS expression in rat cortex with acute multicerebral infarction
The MARCKS and p-MARCKS protein expression were surveyed by western blot technic. MARCKS and p-MARCKS protein were expressed in 1h after ischemia group (M1h), 6h after ischemia group (M6h), 24h after ischemia group (M24h), 72h after ischemia group (M72h), 7d after ischemia group (M7d) vs. Nomal group (CONT), Sham control group (SH) . The changes of MARCKS and p-MARCKS protein expression are identical. After acute ischemic, the expression are all increased, the expression of 1h start heighten, the expression of 24h is supreme, form dynamic changes. The expression of 24h obviously super other group (p<0.01) (Figure 3).
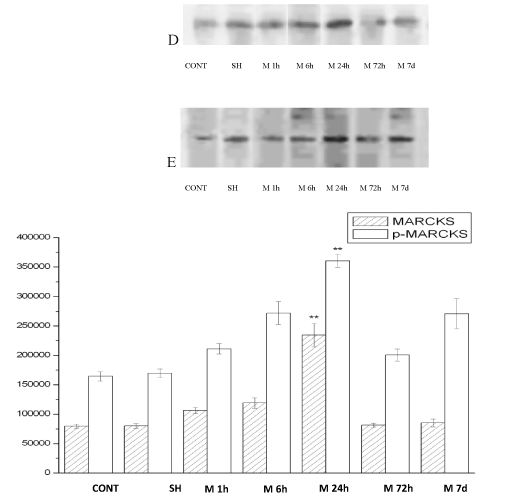
Figure 3. The MARCKS(D)、p-MARCKS (E) expression in rat cortex with acute multicerebral infarction .( ± s, n=6)
The neuropretection of Qingnao drop pilula to cerebral ischemia damage
The MARCKS and p-MARCKS protein expression were surveyed by western blot technic. In hippocampus and contex, MARCKS and p-MARCKS protein were expressed in model group vs. other groups (P<0. 01), in hippocampus CTM group vs. western medicine (WM) group (P>0.05), in contex CTM group vs. WM group (P<0.01) (Figure 4).
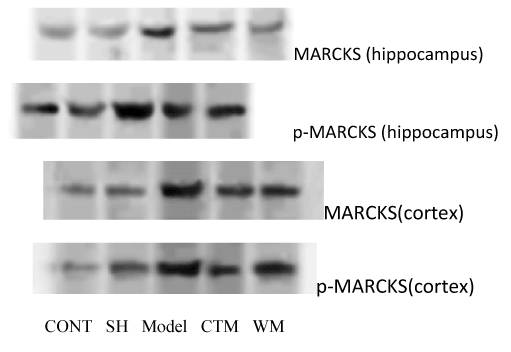
Figure 4. The neuropretection of Qingnao drop pilula to cerebral ischemia damage.
Discussion
The rat Hippocampus and cortex are vulnerable areas of cerebral ischemia. By using western blot, the phosphorylation expression of MARCKS in the hippocampus and cortex was detected after acute cerebral ischemia. After acute ischemic, the MARCKS and p-MARCKS protein in the hippocampus and cortex over-expressed at each time point, over-expression of 1h started heighten, the expression of 24h was supreme, the expression of 72h and 7d slightly decreased, formed dynamic alteration. The MARCKS overexpression in ischemia-vulnerable areas was induced by acute cerebral ischemic injury. The MARCKS overexpression activated PKC, and furtherly promoted its substrate protein MARCKS phosphorylation, and led to PKC-MARCKS signal transduction abnormalities, the cell effects was prolonged. It was seen that at each time point after acute ischemia, there was a positive correlation between the MARCKS and p-MARCKS protein expression and abnormal degree of PKC-MARCKS signal transduction, also damage degree of brain issue with acute cerebral ischemia.
MARCKS was an important protein in cell membrane and cytoskeleton, belonged to membrane skeleton cross-linking proteins. The membrane-bound MARCKS was the most important biological characteristics. MARCKS shows tissue-specific expression with high levels in certain brain regions [6]. When the acute cerebral ischemia happened, some brain neurotransmitters, neuropeptides, and cytokines would be the occurrence of stress-induced changes. The binding between extracellular signaling proteins as ligands and receptors on the cell membrane led to the double polymerization of receptor molecules, activated the G-binding protein receptors, activated phosphoinositide signal transduction pathway, generated the second messenger diglyceride (diacylglycerol, DG), and furtherly activated the PKC-MARCKS signal transduction system, generated a corresponding target effects.
Phosphorylation of MARCKS by PKC, or CaM binding, results in the release of MARCKS from the plasma membrane into the cytoso l [7]. This membrane to cytosol shuttling, or bi-lateral translocation of MARCKS, has been associated with the reorganization of the actin cytoskeleton [8]. After the N-terminal domain of receptors detected the environmental stimulation, the C-terminal histdine kinase of MARCKS was activated, a conservative histidine residue of itself was phosphorylated by means of ATP as a phosphate donor, this phosphate group was passed to the second part which was an aspartic acid residue sited in the cytoplasm of N-terminal response regulator receiver domain, activated the effector of C-terminal histdine , mediated the behavior change of cells.
As previously stated, we have demonstrated that 1 The cell death process could be regulated by different extra and intracellular signals. The activity of signal transduction pathway of PKC-MARCKS mediated by MARCKS was related with the degree of acute cerebral ischemic injury. It should be further examined whether the active change of MARCKS should be the initial factor of acute cerebral ischemic, signal transduction pathway of PKC-MARCKS directly mediated the degeneration and necrosis of nerve cells. 2 The protein phosphorylation process was reversible, reversible protein phosphorylation was an important adjustment method by it cells continuely responded to external signals. It should be further studied that in the process of acute cerebral ischemia, how the role of phosphorylation was, and whether the decreased level of MARCKS and p-MARCKS expression was resulted from phosphorylation. 3 The expression of MARCKS protein was down-regulated by effective ways which played a neuroprotective role in acute ischemia. The suppression of MARCKS gene transcription and the use of protease-activated drug for neuroprotection had a greater significance.
Qingnao drop pilula is composed by notoginseng, gardenia and borneol and it has the therapy of removing toxic substance, dredging collaterals, dispersing brain, inducing resuscitation. It was tested and verified through a long period of clinical trials. Our study showed that Qingnao drop pilula could reduce the injury of brain tissue, MARCKS protein expression, intervene the PKC-MARCKS signal transduction system, delay the process of the death of neuronal cell, thus reduce the acute cerebral ischemia damage. It verified that therapy of removing toxic substance, dredging collaterals, dispersing brain, inducing resuscitation, can have neuropretective effect by interventing PKC-MARCKS signal transduction pathway.
2021 Copyright OAT. All rights reserv
Experimental procedures
Experimental animals and divide into groups
119 male Wistar rats, weighted (340 ± 25) g, were provided by Beijing weitong lihua experimental animal technical co. , LTD. , license number: SCWK (Beijing), 2002-2003. All rats were divided into 7 groups at random: normal group, sham control group, 1h-, 6h-, 24h-, 72h- and 7d after ischemia group, 17 rats in each group.
Model preparation
Injecting the microembolus to establish acute infarction model in rat (improved Kaneko method [9]) : the blood from the left ventricular was withdrawn and retained for 4 hours at 80℃ incubator. The clot was crushed and sifted in 200 μm sieve, 1 mg thromboembolus was put in polyethylene tube filled with 0. 5ml saline and shaked into the suspension.
The rats was anesthetized with 10% chloral hydra(0. 35 ml/100 g)injected in peritoneal cavity. Under the operating scope by a midline incision, the muscles was peeled, the right carotid artery, the right external carotid artery and the right internal carotid artery were isolated. The right common carotid artery were temporarily clamped by using a curved microvascular clip, the 0.3 ml embolus fluid was injected in the right external carotid artery, meanwhile, the microvascular clip was removed so that thromboembolus was sent to brain arteries, then the right internal carotid artery was ligated, the wound was closed. The sham-operated group was operated with the same method as above, the same amount of saline injected. The normal group, sham-operated group, model group, Rats were killed at 24h after model established. The hippocampus and cortex at affected side were frozen in liquid nitrogen.
Duplicating model and sampling method
Nomal group and sham control group were given synchronization observation, exeuted after 72h sampling. 1h after ischemia group, 6h after ischemia group, 24h after ischemia group, 72h after ischemia group, 7d after ischemia group were exeuted and sampled in building 1h, 6h, 24h, 72h, 7d. The method of histopathological draw materials been seen 5. 1 lens samples production method. of The rats which extracted total RNA were taken thier brain on the ice (the whole process should be completed within 5 minutes), taken affected side hippocampal and cortex kept in liquid nitrogen.
The scores of neurological function deficits
The Bederson neurological grading system [10] was used to asses the neurobehavioral outcome of the animals.
Rats were held gently by the tail, suspended one meter above the floor, and observed for forelimb flexion. Normal rats extend both forelimbs toward the floor. Scoring method is as follows: Grade 0, Rats extended both forelimbs towards the floor and that had no other neurological deficit. Grade 1, Rats with infarction consistently flexed the forelimbs contralateral to the injured hemisphere; posture varid from mild wrist flexion and shoulder adduction with extension at the elbow to severe posturing with full flexion of wrist, elbow, and addcuction with any amout of consistent forelimb flexion. Grade 2, Rats were placed on a large sheet of soft, plastic coated paper. With the tail held by hand, gentle lateral pressure was applied behind the rat’s shoulder until the forelimbs slid one meter. The maneuver was repeated several times in each direction. Normal or mildly dysfunctional rats resisted sliding equally in both directions. Severely dysfunctional rats had consistently reduced resistance to lateral push toward the paretic side, or appeared circling behavior. Grade 3, Rats were conscious, but their bodys falled to the left side. Grade 4, Rats were confusion, and no spontaneous activity. Grade 5: Rats were dead. The whole neurologic examination can be performed in 3-5 min.
Preparation of lens tissue samples
The rats were anesthetized intraperitoneally, and perfused their hearts with physiological saline and heparin sodium, then perfused and fixed with 4% paraformaldehyde. The whole brain was removed when the rat’s body stiff, then continue fixed in 4% paraformaldehyde at least 24h. The brain ( from the forebrain frontopolar to occipital lobe) was chilled and coronally sectioned into 2-mm-thickness slices, then ethanol dehydrated conventionally, paraffin embedd, serial section (4 microns), HE stained.
Westen blot
Total protein extraction: rats were decapitated and brain tissues were taken out in five minutes. The hippocampus tissue at affected side was put in homogenate buffer (1g:10 ml), ground and homogenated at 4℃. The protease inhibitor PMSF (10 mM) was added in the buffer. The homogenate buffer was put aside for 30 minutes at 4℃, oscillated three times, each time for 10 seconds. The homogenate was centrifuged at 13, 000 rpm for 20 min at 4℃. The supernatant was collected and transferred new tube. The content of protein in dissolve fluid was treated with protein test kit, stored respectively at -80℃.
SDS-PAGE electrophoresis and western blotting: The gel solution was prepared with 5% laminated plastic and 8 ℅ separating gel mixture. The sample of protein was mixed with 3X sample buffer in the ratio of 1:2, boiled at 98℃ for 5 minutes, loaded 7 ug protein per gel well. Electrophoresis was performed at a constant current of 20 mA for 4h. The sample was transferred at a steady current of 0. 65 mA per square meters according to gel square for 2h.
After blocking with 5% Blotto TBS-T solution overnight at 4℃, the membranes were incubated with primary MARCKS antibodies (diluted 1:2000) and pre-MARCKS antibodies (diluted 1:1000), shaked on the ice for 2h. The membrane was washed thrice (15 minutes each) with TBS-T, and incubated horseradish peroxidase conjugated donkey anti-goat IgG antibody (diluted 1:2000, 1:1000), rocked for 1 h at room temperature. Developing: An equal amount of ECL A and B fluid was mixed according to 0. 125 ml/cm2 membrane area. The NC membrane was immersed in TGM, repeatedly oscillated, taken out after 30 seconds, then put in the dark room of GENE GNOME SYSTEM BIO IMAGING. After adjusting the exposure time, the images photoed by using GeneSnap software were saved. The optical density value of brand was measured by means of GeneTools sofgware (SynGene), and the optical density value represents the relative expression levels of MARCKS protein.
Acknowledgments
This research was supported by the Ministry of Science and Technology of the People’s Republic of China (No. 2012CB518406).
References
- Bai T, Luoh SW (2008) GRB-7 facilitates HER-2/Neu-mediated signal transduction and tumor formation. Carcinogenesis 29: 473-479. [Crossref]
- Yang TT, Wang SJ (2008) Facilitatory effect of glutamate exocytosis from rat cerebrocortical nerve terminals by alpha-tocopherol, a major vitamin E component. Neurochem Int 52: 979-989. [Crossref]
- Laura E Ott, Eui Jae Sung, Adam T, Melvin, et al. (2013) Fibroblast Migration Is Regulated by Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) Protein. PLOS ONE 8: e66512. [Crossref]
- Tzlil S, Murray D, Ben-Shaul A (2008) The "electrostatic-switch" mechanism: Monte Carlo study of MARCKS-membrane interaction. Biophys J 95: 1745-1757. [Crossref]
- Tsaadon L, Kaplan-Kraicer R, Shalgi R (2008) Myristoylated alanine-rich C kinase substrate, but not Ca2+/calmodulin-dependent protein kinase II, is the mediator in cortical granules exocytosis. Reproduction 135: 613-624. [Crossref]
- Thomas Theis, Bibhudatta Mishra, Maren von der Ohe, et al. (2013) Functional Role of the Interaction between Polysialic Acid and Myristoylated Alanine-rich C Kinase Substrate at the Plasma Membrane. J Biol Chem 288: 6726-6742. [Crossref]
- Swierczynski SL, Blackshear PJ (1995) Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein.mutational analysis provides evidence for complex interactions. J Biol Chem 270: 13436-13445. [Crossref]
- Yarmola EG, Edison AS, Lenox RH, Bubb MR (2001) Actin filament crosslinking by MARCKS:Characterization of two actin-binding sites within the phosphorylation site domain. J Biol Chem 276: 22351-22358.
- Kaneko D, Nakamura N, Ogawa T (1985) Cerebral infarction in rats using homologous blood emboli: development of a new experimental model. Stroke 16: 76-84. [Crossref]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, et al. (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17: 472-476. [Crossref]




