Purpose: To determine whether a second induction course (12 weeks) of intravesical BCG improves response and 2-year recurrence rate compared to standard initial 6-week induction BCG therapy in non-muscle-invasive bladder cancer patients.
Materials and methods: One hundred thirty-two patients with high-risk Ta, T1 and CIS underwent a restaging TUR and were treated with 6-week induction BCG therapy. Thirty-eight patients had persistent tumor after 3 months and received a second 6-week induction course. All patients were evaluated for response at 6 months and followed 2 years for tumor recurrences.
Results: Complete response after 6 months was marginally better in patients treated with 2 induction cycles of BCG than one (92% vs. 83%). Tumors recurred in 11% of patients receiving two BCG courses vs. 29% of patients treated with initial 6-week BCG induction (p = 0.03).
Conclusion: A second induction course (12 weeks) of BCG therapy was associated with fewer tumor recurrences over standard 6-week BCG induction alone in selected high-risk patients with non-muscle-invasive bladder cancer.
bladder cancer, BCG
Intravesical BCG is preferred adjuvant therapy after transurethral resection of high grade non-muscle invasive bladder cancer. A 6-week induction course of BCG therapy results in a complete response rate of 70% after 3 months [1]. Achieving a complete response to BCG is critical to disease-free survival [2]. Not all patients respond to the initial BCG induction course, suggesting that a second induction course may be needed. Some studies show 40% to 60% of patients who do not respond to initial induction by 3 months respond to a second cycle of 6-weekly instillations after 6 months [1,3], however others show repeat BCG induction does not improve clinical outcomes [4-6].
Patients with preexisting BCG-specific immunity, as measured by positive PPD skin test [7,8] or BCG-specific T-cells [9], have longer disease-free survival than BCG-naïve patients. This suggests that boosting local immunity may improve initial response rates to BCG therapy. Intravesical BCG alone stimulates a systemic immune response [10,11], and we showed that one 6-week induction course of BCG could convert a negative tuberculin skin test to positive [7]. This provides a rationale for a second induction course of BCG in selected patients. This study compares the short-term outcomes of high-risk bladder cancer patients receiving either one or two induction courses of BCG therapy. The hypothesis is first exposure to BCG primes the immune system to enhance antitumor effect of subsequent BCG therapy.
After obtaining institutional review board approval, BCG-treated patients from 2011 to 2014 were retrospectively reviewed. Consecutive patients with multiple, high grade non-muscle invasive bladder cancer underwent contemporary maximum re-staging transurethral resection (TUR) to remove residual disease, followed 2 to 3 weeks later by induction (6 weeks) BCG therapy. They were evaluated for initial response at 3 months. Patients with persistent Ta or carcinoma in situ (CIS) received a second 6-week induction course, evaluated after six months by cystoscopy, biopsy and urine cytology and followed for full two years. Patients with T1 tumors after BCG were excluded from analysis. Maintenance BCG was not used because a prior randomized trial failed to benefit our high-risk patient population [7].
End points were response to BCG at 6 months and 2-year disease-free survival in patients who had a complete response after one or two induction cycles. A complete response to BCG was defined as no evidence of disease on cytoscopy, biopsy and urine cytology. Basic descriptive statistics were used to determine differences among patients, frequency of recurrences (Chi-square) and tumor-free survival times (log-rank).
One hundred thirty-two patients with high grade Ta, T1 and CIS tumors were evaluated by re-staging TUR and treated with induction (6 weeks) intravesical BCG therapy. Ninety-four patients (72%) achieved a complete response to BCG at 3 months and were followed without additional BCG treatments. Thirty-eight patients (28%) had persistent CIS after 3 months and received a second 6-weeks BCG induction course. All patients were followed every 3 to 6 months for two years. PPD skin tests were not measured before or after BCG therapy.
Of the 132 patients receiving one or two induction BCG courses, the median age was similar (70 and 69 years, respectively), and 72% in both groups were men. Fifty-three percent of patients who had one or two BCG cycles had TaHG tumors, 47% had T1HG tumors and 78% in both groups (103 cases) had associated carcinoma in situ.
Overall, 113 of the 132 patients (85.6%) achieved a complete response to BCG at 6 months. Of the 94 patients receiving one BCG induction, 78 (83%) maintained complete responses after 6 months vs. 35 of 38 patients (92%) who achieved a complete response after two BCG inductions (odds ratio = 2.3 (95% CI, .65-8.7), p = 0.17). With 2-year follow-up, 29% (27 cases) of 94 patients recurred after a single induction BCG versus 11% (4 cases) of the 38 patients receiving two induction cycles (Odds ratio = 0.29 (95% CI, 0.09-.90), p = 0.03). The Figure shows 2-year disease-free survival of patients who achieved a complete clinical response after 6 months to either one (78 cases) or two (35 cases) BCG induction courses. Recurrence-free survival time favored the group after second BCG induction (90% vs. 71%; p = 0.03). Median survival was not reached in either group (mean recurrence-free survival time 23 months after two BCG courses vs. 20 months after one course).
Tumor progression was not a primary endpoint, however none of the patients progressed after receiving a second induction vs. 4% (4 of 94) after one BCG induction.
The major finding of this study is that bladder tumor patients with persistent disease 3 months after initial induction BCG who received a second induction course had higher complete response rates by 6 months, fewer tumor recurrences over 2 years, and longer disease-free intervals than similarly-staged patients after single induction BCG therapy. Although 6-month responses were marginally higher after second induction, tumor-free survival was superior in patients with residual non-invasive disease than patients who responded to initial BCG therapy and did not receive second induction. Although another TUR to remove persistent tumor at 3 months may have improved the 6-month complete response rates [12], the second BCG induction course is more likely responsible for the longer 2-year recurrence free survival time.
There are weaknesses inherent in this pilot study. It is a retrospective analysis of a single surgeon, single institution experience, involving small numbers of high-risk patients followed for a short time. Baseline patient differences may have biased results. PPD skin tests were not measured to correlate systemic immunity with subsequent BCG outcomes. Maintenance BCG was not given, however our outcomes in patients with induction BCG alone are similar to published results using maintenance BCG [13].
The results should be interpreted with caution since we have shown that up to 20% of patients who have positive urine cytology 3 months after a single BCG induction may convert to complete responses after 6 months without additional BCG therapy [14], indicating actual benefit from a second induction cycle of BCG (and in which patients) remains unproved. However, the novel finding of this pilot study is not that two cycles of BCG are better than one, but tumor-free intervals after a second intravesical BCG course in first nonresponders was superior to patients who had responded initially to first exposure to BCG therapy. This suggests that prior sensitization to BCG may enhance local anti-tumor immune response to subsequent BCG exposure. Accordingly, we have launched an ongoing prospective trial to test whether two induction courses of BCG will improve complete responses and early recurrence rates in all patients with high grade non-muscle-invasive bladder cancer.
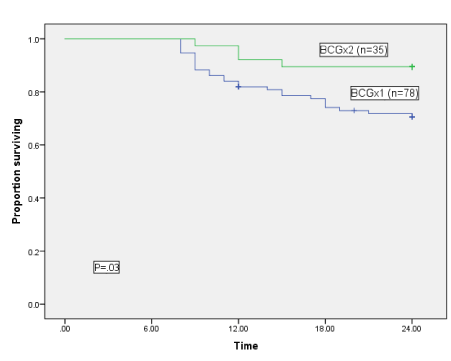
Figure 1. 2-year recurrence-free survival among complete responders at 6 months after one (BCGx1) versus two (BCGx2) 6-week induction BCG courses.
A second induction course of BCG therapy appears to reduce the frequency of tumor recurrences over standard 6-week BCG induction alone in selected high-risk patients with non-muscle-invasive bladder cancer.
- Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, et al. (2000) Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol 163: 1124-1129. [Crossref]
- Lerner SP, Tangen Cm, Sucharew H (2009) Failure to achieve a complete response to induction BCG therapy is associated with increased risk of disease worsening and death in patients with high risk non-muscle invasive bladder cancer. Urol Oncol 27: 155-9.
- Sylvester RJ, Van der Meijden A, Witjes JA, Jakse G, Nonomura N, et al. (2005) High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology 66: 90-107. [Crossref]
- Gontero P, Bohle A, Malmstrom PU, O'Donnell MA, Oderda M, et al. (2010) The role of bacillus Calmette-Guérin in the treatment of non-muscle-invasive bladder cancer. Eur Urol 57: 410-429. [Crossref]
- Palou J, Laguna P, Millán-Rodríguez F, Hall RR, Salvador-Bayarri J, et al. (2001) Control group and maintenance treatment with bacillus Calmette-Guerin for carcinoma in situ and/or high grade bladder tumors. J Urol 165: 1488-1491. [Crossref]
- Kamat AM, Porten S (2014) Myths and mysteries surrounding bacillus Calmette-Guérin therapy for bladder cancer. Eur Urol 65: 267-269. [Crossref]
- Badalament RA, Herr2021 Copyright OAT. All rights reservomized trial of maintenance versus nonmaintenance intravesical BCG therapy of superficial bladder cancer. J Clin Oncol 5: 441-449.
- Bilen CY, Inci K, Erkan I (2003) The predictive value of purified protein derivative results on complications and prognosis in patients with bladder cancer treated with BCG. J Urol 169: 1702-1705.
- Biot C, Rentsch CA, Gsponer JR, Birkhäuser FD, Jusforgues-Saklani H, et al. (2012) Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci Transl Med 4: 137ra72. [Crossref]
- Brosman SA (1982) Experience with bacillus Calmette-Guerin in patients with superficial bladder carcinoma. J Urol 128: 27-30. [Crossref]
- Taniguchi K, Koga S, Nishikido M, Yamashita S, Sakuragi T, et al. (1999) Systemic immune response after intravesical instillation of bacille Calmette-Guérin (BCG) for superficial bladder cancer. Clin Exp Immunol 115: 131-135. [Crossref]
- Qie Y, Hu H, Tian D (2016) The value of extensive transurethral resection in the diagnosis and treatment of nonmuscle invasive bladder cancer with respect to recurrence at the first follow-up cystoscopy. Onco Targets Ther 9: 2019-25.
- Herr HW, Dalbagni G, Donat SM (2011) Bacillus Calmette-Guérin without maintenance therapy for high-risk non-muscle-invasive bladder cancer. Eur Urol 60: 32-36.
- Herr HW, Badalament RA, Amato DA (1989) Superficial bladder cancer treated with BCG: a multivariate analysis of factors affected tumor progression. J Urol 141: 22-29.

