Improving patient experience after total knee replacement is an important predictor of final outcome. Reducing pain and swelling following surgery will enhance recovery leading to early discharge and increased bed turnover rate. This approach is profitable to both the patient and the hospital.
In our tertiary referral hospital, the current standard practice for post-operative knee replacement surgery is for patients to be entered into an Enhanced Recovery Programme (ERP) [1,2]. This involves a multi-faceted and multi-disciplinary approach in improving pain control, early mobilisation and outcome by means of best practice post op analgesia, intra-operative local anaesthetic infiltration, and early rehabilitation.
A recent addition to ERP is a cryopneumatic device, the ‘Game Ready’ system. Potentially, this device combines the benefit of cryotherapy with dynamic intermittent compression [3]. Cryotherapy can reduce leucocyte migration thus reducing inflammation and slows down nerve signal transmission thus resulting in short term analgesia. On the other hand, compression might help in decreasing oedema and hemarthrosis by increasing hydrostatic pressure and reducing intra-articular volume. The net effect is reduction in pain, oedema and swelling thus enhancing recovery and improving postoperative stiffness.
Although the newer device is appealing with respect to its proposed benefits, literature support on its clinical effectiveness and safety are sparse. Murgier and Cassard studied the utility of this ‘Game Ready’ system following anterior cruciate ligament (ACL) reconstruction against cryotherapy with static compression [4]. The postoperative pain scores were not significantly different but the patients in the ‘Game Ready’ group required fewer analgesics and had improved knee range of motion (ROM). Su et al., conducted a prospective, randomised, multi-center trial in total knee arthroplasty patients comparing ‘Game Ready’ with ‘ice & static compression’ [5]. There was no difference in ROM, function and swelling between the two groups. However, ‘Game Ready’ group had significantly lower amount of narcotic compression in the first two postoperative weeks. The main drawback of this study is that out of the 294 patients randomised, only 187 were available for final analysis giving a drop-out rate of 36.4% for a study where the postoperative follow-up is just 6 weeks. Moreover, an ‘intention-to-treat’ analysis was not performed thus a potential bias could not be ruled out. Their regimen involved treating the patients for two postoperative weeks with either ‘Game Ready’ or ‘ice & static compression’ whereas we employ ‘Game Ready’ only for the time period when the patient is in-hospital.
Our present study proposes to find out if there is a benefit to patients and the trust in using the ‘Game Ready’ cooling compression system as part of the standard care in the Enhanced Recovery programme for total knee replacement surgery.
arthroplasty; knee replacement; cryotherapy; randomised controlled trial
Primary aim
- To determine if there is any benefit in post-op pain control during the Enhanced Recovery Programme by adding ‘Game Ready’ system.
Secondary aims
- To determine if there is any benefit in post-op swelling, stiffness and function.
- To determine if there is reduction in patient length of stay.
- To assess patient satisfaction.
- To identify complications, if any, associated with use of ‘Game Ready’ system.
Study design- Randomised Controlled Trial (RCT).
Study group- Patients managed with Enhanced Recovery Programme with ‘Game Ready’ system.
Control group- Patients managed with Enhanced Recovery Programme without ‘Game Ready’ system.
Place of study- Lower Limb Unit, Wrightington hospital.
Inclusion criteria
- Patients suitable for the Enhanced Recovery Programme following total knee replacement.
- Patients undergoing primary total knee replacement for osteoarthritis of knee.
- Patients aged between 40 to 90 years.
Exclusion criteria
- Patients with inflammatory joint disease such as rheumatoid arthritis.
- Patients not suitable for Enhanced Recovery Programme (ex: Ischemic heart disease, chronic kidney disease).
- Patients undergoing complex primary or revision knee replacement.
- Patients with chronic painful conditions affecting knee apart from osteoarthritis (ex: Complex Regional Pain Syndrome).
- Patients with contra-indication to dynamic compression therapy:
- Who are in the acute stages of inflammatory phlebitis in the affected region.
- Who have any history of deep vein thrombosis (or pulmonary embolus) in the affected region (to be treated with this therapy).
- Who have significant arteriosclerosis or other vascular ischemic disease in the affected region
- Who have a condition in which increased venous or lymphatic return is not desired in the affected extremity (ex: carcinoma).
- Who have decompensated hypertonia in the affected region.
- Patients with contra-indication to cryotherapy:
- Who have significant vascular impairment in the affected region (ex: from prior frostbite, diabetes, arteriosclerosis or ischemia).
- History of acute paroxysmal cold hemoglobinuria or cryoglobulinemia.
Sample size estimation
The primary endpoint of interest is the analgesic requirement measured as the morphine equivalent. Sample size calculations are based on the previous results of Su et al., [3]. The difference in morphine equivalent dose between the two groups is 171mg. Here results suggest skewed distributions which indicate that data should be analysed on the log scale. An estimated standard division of 1.2 is obtained using the statistical rule of thumb of range/4. Using a two-sided alpha level of 0.05, collecting data on 100 patients (50 in each arm), this study will be powered at the 80% level to detect a log adjusted difference of 0.7.
Statistical analysis
Continuous data shall be summarized as medians with associated inter-quartile ranges. Categorical data are summarized as frequencies of counts with associated percentages.
The change in morphine equivalent will be compared across groups using a t-test. Appropriate transformations (i.e., a log transformation) will be applied to account for the anticipated skewed nature of the data. Further analysis using linear regression techniques will be used to adjust for prognostic factors of importance.
Analyses of secondary endpoints will follow the same approaches as the primary outcome with the exception of post-operative swelling or stiffness which will be measured as a categorical variable and shall be compared across treatment groups using a Chi-Square/Fishers test as appropriate.
Primary outcome measure:
- Analgesic requirement (in morphine equivalent) over and above the standard ERP allowance during in-patient stay.
Secondary outcome measure:
- Pain- Visual Analogue Scale (1 to 10).
- Swelling- Measurement of limb girth (Level of knee, 5cm above knee, 5cm below knee).
- Stiffness- Range of motion (using goniometer).
- Function- Visual Analogue Scale (12021 Copyright OAT. All rights reservTime to up and go test (TUG).
- Complications:
- Wound related- blistering around wound, wound discharge, early postop infection, etc.
- Analgesic intake related- constipation, nausea, vomiting, etc.
- Others- foot drop, deep vein thrombosis.
- Number of premature discontinuation of ‘Game Ready’ system usage (if any) and reasons for discontinuation.
- Patient satisfaction- Visual Analogue Scale (1 to 10), Questionnaire to ‘ERP with Game ready’ group, Hospital Anxiety and Depression scale (HAD).
- Duration of in-hospital stay.
Patients who fulfil the inclusion and exclusion criteria will be identified by the clinician and referred to another independent trial investigator. The independent trial investigator will then invite the patient to participate in the study and provide them with the patient information sheet. Patients approached for inclusion will not be required to undergo any additional blood tests, invasive procedures or extra outpatient appointment follow-up other than that required for standard post-operative knee replacement care.
Those who are willing to participate will be consented for participation by the independent trial investigator. This consent for the study will be separate from the consent obtained for knee replacement procedure. Patients will then be randomised into one of the two groups- ‘Routine ERP’ and ‘Game Ready ERP’. Randomisation will be performed according to computer generated random table. The description of the recruitment and consent process is further depicted in Figure 1.
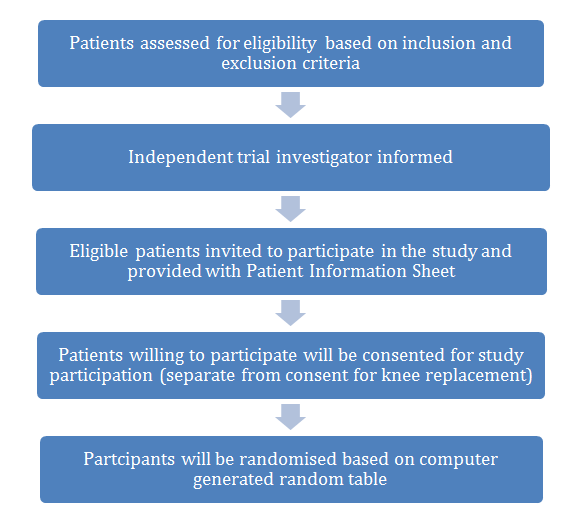
Figure 1.Description of recruitment and consent process.
All patients will complete a preoperative questionnaire and clinical assessment. Both sets of patients will have their circumferential measurements conducted pre op at the level of the knee and 5cm above and below. This will then be assessed again at day 2 and at week 6 to assess and differences in swelling post operatively. Following surgery, operative details will be recorded including tourniquet time, blood loss and implants used. Patients will be subjected to ERP with or without ‘Game Ready’ system depending on the group allocated. Patients will be asked to complete a questionnaire and clinical assessment will be performed 24 hours postop (PO24), 48 hours postop (PO48) and 6 weeks postoperatively (6W). Patients in the ‘ERP with Game Ready’ group will have an additional questionnaire to record patient satisfaction with the Game Ready system. Data on usage of ‘Game Ready’, the temperature and pressure settings employed will also be recorded.
The discharge criteria for patients will be:
- Satisfactory wound and general condition.
- Walk independently with crutches/walking aid.
- Get in/out of bed and on/off the chair/toilet independently.
- Be able to get up/down stairs if required at home.
- Make satisfactory progress in straightening and bending the knee.
- Make satisfactory progress in strengthening the muscles in the operated extremity.
- Satisfactory radiological picture.
- Have all the equipment/help necessary at home.
Whenever there is a delay in discharge, the reason for delay will be noted. All the complications occurring during in-hospital stay and during the first 6 weeks of postoperative period will be recorded. Data will be analysed with the help of an expert biostatistician.
Use of interpreter
All patients who have a poor understanding of the English language consent for surgery via an interpreter either in person or over the phone in keeping with trust guidelines. This is standard practice of care and interpreters could be used at the same opportunity to obtain informed consent from patients for the study.
Adverse events
Should any adverse events occur during the study timeframe all participants would be immediately informed. In case of serious adverse events/ patient harm, the trial will be terminated prematurely and all study participants will be notified.
Confidentiality and data handling
All researchers are GCP (Good Clinical Practice) trained and adhere to the storage of health records policy and have undertaken mandatory corporate training in data protection. The NHS code of patient confidentiality will be adhered to along with the data protection act.
Once the data has been analysed and research published the data will be deleted from NHS encrypted computers. We envisage this will take 12 months.
- Dwyer AJ, Thomas W, Humphry S, Porter P (2014) Enhanced recovery programme for total knee replacement to reduce the length of hospital stay. J Orthop Surg 22: 2. [Crossref]
- McDonald DA, Siegmeth R, Deakin AH, Kinninmonth AWG, Scott NB (2012) An enhanced recovery programme for primary total knee arthroplasty in the United Kingdom-follow up at one year. Knee 19: 525-529. [Crossref]
- Su EP, Perna M, Boettner F, Mayman DJ, Gerlinger T, et al. (2012) A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br 94(11 Supple A):153-156. [Crossref]
- Murgier J, Cassard X (2014) Cryotherapy with dynamic intermittent compression for analgesia after anterior cruciate ligament reconstruction. Preliminary study. Orthop Traumatol Surg Res100: 309-312. [Crossref]
- Hollman JH, Beckman BA, Brandt RA, Merriwether EN, Williams RT, et al. (2008) Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J Geriatr Phys Ther 31: 53-56. [Crossref]

