Traumatic Brain Injury (TBI) is defined as an injury to the brain parenchyma resulting in neurological deficit following a trauma. Cell transplantation has evolved as a novel treatment modality in the management of TBI. Autologous Bone Marrow Mononuclear Cells (BMMNCs) have shown beneficial effects in the functional recovery of neurological deficits. We present a case of a 15 year old female who suffered from a TBI due to a road traffic accident, 7 years ago. She underwent two doses of intrathecal transplantation of autologous BMMNCs for chronic TBI. No severe adverse events were noted. She showed improvements in her speech, cognitive abilities, memory, higher function and fine motor skills. PET CT scan was used as a monitoring tool to evaluate the effect of cellular transplantation at a cellular level. On comparing the PET CT scan brain performed 6 months after the intervention with the previous scan, increased metabolic activity in various areas of the brain was observed. These changes also correlated to the functional improvements observed in the patient. Hence, stem cell transplantation may have a promising future as a therapeutic intervention in chronic TBI.
traumatic brain injury, autologous bone marrow mononuclear cells, cellular transplantation, stem cells, cell transplantation, PET CT scan brain
Traumatic brain injury (TBI) constitutes a leading public health and socioeconomic problem across the globe [1]. TBI is defined as an ‘alteration in brain function, or other evidence of brain pathology, caused by an external force’ [2]. It poses a potential threat to the brain parenchyma following head trauma [3], thus resulting in neurological deficit.
It is considered to be a ‘silent epidemic’ as there is lack of awareness about the magnitude of the consequences [4]. It is estimated that more than 50% of TBI’s are caused due to motor vehicle accidents. The other causes of TBI could be because of assaults, blasts, impact sports, falls etc. [5]. TBI is commonly associated with impaired attention span, mood disorder, poor decision making as well as physical disabilities [4].
Cerebral injury after TBI leads to direct trauma to the tissue and alters the regulation of cerebral blood flow and metabolism. This in turn triggers a cascade of neurochemical changes thus leading to apoptosis [6]. Due to inadequate treatment modalities in chronic TBI, it is imperative to focus on a multidisciplinary approach. Cellular transplantation has recently gained attention as a therapeutic strategy for various neurological disorders including TBI. The aim of this novel intervention is to cease the degeneration and replace the damaged neurons. Stem cells have a unique potential of differentiating and multiplying into mature cells, thus replacing the damaged cells [7,8]. Various preclinical studies have suggested the safety and efficacy of stem cells in TBI [9-11].
We present a case of 15 year old female who suffered a TBI due to a road traffic accident. She underwent intrathecal transplantation of autologous BMMNCs; 7 years post TBI to overcome the residual deficits due to injury.
A 15 year old female had TBI, 7 years prior to cell transplantation following a road traffic accident. TBI was associated with transtentorial herniation and diffuse axonal injury. Decompressive craniotomy and evacuation of hematoma was the immediate line of management. At the time of acute injury, MRI showed hemorrhagic contusion involving bilateral parieto-occipital, posterotemporal, posterior frontal and anterior temporal regions with tiny areas of hemorrhagic contusions noted in the cortical, sub cortical and bilateral cerebellar hemispheres. She was in coma for one week and was on mechanical ventilation; after which she recovered. Speech recovered in 3-6 months and standing in 8-12 months. She was on rigorous rehabilitation for a year.
7 years later she underwent cellular transplantation. On evaluation before intervention, she presented with right sided hemiparesis and right sided neglect. She was normotonic and had intact sensations. Her superficial reflexes were diminished whereas deep reflexes such as the knee and the ankle jerk were brisk. Her bowel and bladder functions were normal. She had developed a right foot drop with right external rotation. No wasting was noted. Her voluntary control was affected in the right upper and lower extremity (fair) and hand function was also affected. Behavior was aggressive and adamant. She had a low pitch speech. Her fine motor co-ordination was also affected and was unable to carry out fine motor tasks such as buttoning-unbuttoning and writing. Her dynamic standing balance was fair. Functionally, she was independent in all her ADLs. Her functional independence measure (FIM) score was 102. Memory was grossly affected with partial affection of vision. She could not identify objects but could recognize her family members. On Mini-Mental State Examination (MMSE) she scored 11 indicating moderate cognitive impairment Brain MRI was suggestive of encephalomalacia with gliosis. PET CT scan revealed gliotic areas in bilateral parieto-occipital & right anterior inferior temporal region with reduced FDG uptake.
The patient selection and the intervention protocol were based on the World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects [12]. The ethical approval for the intervention was obtained from Institutional Committee for Stem Cell Research and Therapy (IC-SCRT). The procedure was explained to patient's parents and a dully filled informed written consent was obtained from them. She was thoroughly assessed by a team of expert doctors prior to the intervention. Pre-surgery routine blood tests, urinalysis and chest x-ray were carried out for surgical and anesthetic fitness. 300 mcg of Granulocyte colony-stimulating factor (G-CSF) injections were administrated 72 hours and 24 hours prior to BMMNC transplantation. This enhances the mobility of BMMNCs and stimulates CD34+ cells. With the patient in supine position, local anesthesia was given in the region of anterior superior iliac spine. 100 ml of bone marrow was aspirated from the iliac bone with the help of a bone marrow aspiration needle and collected in heparinized tubes. Separation of mononuclear cells was carried out by the density gradient method under aseptic conditions in the stem cell laboratory. Mononuclear cell (MNC) count and viability was performed. Enumeration of CD34+ cells was done by FACS analysis. 8.6×107 cells with viability of 96% were administered intrathecally at the level of L4-L5 using a 25 G spinal needle. 1 gm of methyl prednisolone in 500 ml Ringer’s Lactate (RL) was simultaneously injected intravenously to alleviate local inflammatory response and increase the stem cell survival. She was closely monitored during her stay at the hospital for any immediate adverse events. Post transplantation, the patient underwent extensive neurorehabilitation including physical therapy, occupational therapy, speech therapy, psychological counseling and speech therapy. Home exercise program was also prescribed to her. The patient was followed up at regular intervals.
One year later, she underwent a second dose of cellular transplantation. The protocol for which remained same.
At 3 months follow up, FIM score improved from 102 to 110. Gross motor and fine motor activities with the right hand improved. Grip and opposition to all the fingers, balance and sitting tolerance also improved. Higher functions such as memory, concentration, speech and attention increased. Emotional outbursts reduced considerably. Her social interaction and confidence also showed improvement. Cognitive ability to follow instructions and take responsibility for the tasks increased.
6 months after intervention, due to the improvements in her speech, cognitive abilities and increased social interaction she started attending regular school. Concentration, attention span, fine motor tasks further improved. Anger outbursts and unnecessary laughing episodes reduced. But, her fatigability increased. MMSE scores improved from 11 to 14.
PET CT scan brain was repeated 6 months after transplantation. On comparing it to the scan performed before transplantation, an increased metabolic uptake in anterior cingulate cortex, posterior cingulate cortex, mesial temporal regions which include hippocampus and amygdala and sub-cortical regions which include basal ganglia, pons and cerebellum was recorded (Figure 1).
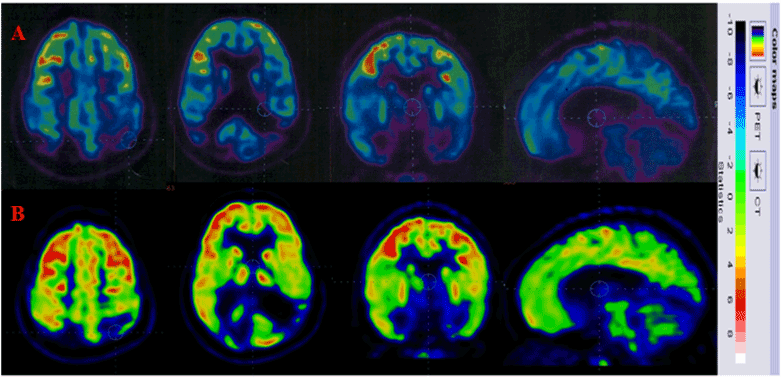
Figure 1. Row A indicates the FDG uptake prior to cellular transplantation. Row B indicates increased FDG uptake post cellular transplantation.
One year after the intervention, her endurance and sitting tolerance improved. Higher cognitive functions such as short term memory, formation of new memory, understanding abilities also improved. Her anger outbursts and adamant behavior showed significant improvement. Due to her improved vision, she was able to perceive things clearly. Fine motor activities like zipping –unzipping, buttoning-unbuttoning also improved. She was able to use both the upper extremities efficiently. Fine motor co-ordination also increased. Right sided neglect decreased.
In view of these significant improvements, she underwent 2nd dose of cellular transplantation. 5 months after the 2nd transplantation, all her improvements including vision, eye contact, social interaction, cognitive abilities, attention span, recent memory, awareness, multitasking abilities, speech, communication, gross and fine motor activities were maintained since her last follow up. Behavioral issues further improved. She was much calmer as compared to before.
Cerebral injury after TBI causes ischemia of the cerebral tissue, thus causing anaerobic glycolysis. These events lead to the membrane degeneration of vascular and cellular structures of the cerebral tissue, thus resulting in necrosis and apoptosis [6]. Decompressive craniotomy [13], diverse pharmacological drugs are proposed in the management of TBI [14]. However, these therapeutic interventions do not halt the disease process.
Cell transplantation has a unique potential to alter the course of the disease, as stem cells have the ability to differentiate and self renew into mature, multipotent cells [8]. It has gained popularity in the management of various neurological disorders such as ALS, stroke, Alzheimer, spinal cord injury, and TBI [15-19]. Preclinical studies [9-11] have used various types of stem cells and different routes of administration in the treatment of TBI. These studies suggest that cell transplantation may improve functional outcomes after TBI.
In this study, we administered autologous BMMNCs intrathecally to the patient. BMMNCs were used as they are safe, can be easily isolated and do not have any ethical issues. To reduce the complications of cell rejection, graft vs. host disease, an autologous approach was preferred. The mechanism of action of BMMNCs is twofold; to protect the existing tissue and to replace the damaged tissue. In TBI, there is diffused axonal injury and the myelin sheath is disrupted, thus affecting the neurotransmission [20]. Stem cells migrate and home onto the injury site, thereby reducing the inflammation by mediating inflammatory markers [21]. These cells also differentiate and proliferate into neural cells, oligodendrocytes which cause remyelination of the damaged axons and enhance neural pathways [22]. Neuroprotection and neuroangiogenesis is brought about by the secretion of various factors such as a brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) by these stem cells [23].
Many clinical studies are being researched across the globe, which suggest the safety and efficacy of cellular therapy in TBI. However, the targeted route of transplantation of stem cells is of prime importance. Higher concentration of stem cell in the target area is desirable for maximizing the benefits of cellular transplantation. Hence, regional routes of transplantation of cells should be chosen [24]. In TBI, the ideal targeted route of transplantation maybe intracerebral, but it is an invasive procedure which may further lead to secondary damage to the cerebral tissue. In this case study, the patient underwent intrathecal transplantation of autologous BMMNCs. This route is minimally invasive and it targets the desired area. Intrathecal delivery of stem cells increases neural connectivity, decreases pro inflammatory mediators in the brain and spinal cord and enhances migration and differentiation of neuronal precursors [25].
Various studies also discuss about the intravenous transplantation of autologous BMMNCs [21,26]. But, intravenous transplantation may trap the delivered cells in the lungs and the total number of cells reaching the target area may not be adequate to produce desirable results [27].
Our previously published research article suggests that cellular transplantation promotes functional recovery of neurological deficit, thus leading to an improved quality of life in chronic TBI [28]. In our current case report, the patient showed improvements in her speech, cognitive abilities, attention span, concentration, recent memory, fine and gross motor activities. Her level of independence also improved as the FIM score increased from 102 to 107. MMSE scores improved from 11 to 14 indicating improved cognition. The PET-CT scan brain also showed changes in brain metabolism corresponding to the symptomatic improvements. PET CT scan brain is diagnostic imaging tool which aids in measuring the brain glucose metabolism using tracer (18F) fluorodeoxyglucose (FDG). Decreased metabolism is suggestive of decreased function of the neurons. An increased uptake of FDG indicates increased metabolism which is implied as improved functioning of the neurons [29,30]. In this case, post cellular transplantation, there was increased uptake of FDG in the singulate gyrus, amygdala, frontal and temporal lobes which substantiates improvements observed in learning, memory, emotions, social interaction and vision.
2021 Copyright OAT. All rights reserv
This case study showed that cell transplantation holds a promising future in the management of TBI. It has the potential to preserve the viable neurons and replace the damaged ones by the mechanism of neuroprotection and neuroangiogenesis. Cellular transplantation along with neurorehabilitation plays a pivotal role in the functional recovery of chronic TBI patients, thus improving their quality of life. A comparative study of various types of cells and routes of transplantation should be researched in detail. Rigorous methodological trials using randomization, blinding strategies, control groups should be conducted for conclusive findings.
- Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7: 728-741. [Crossref]
- Menon DK, Schwab K, Wright DW, Maas AI; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health (2010) Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 91: 1637-1640. [Crossref]
- Heegaard W, Biros M (2007) Traumatic brain injury. Emerg Med Clin North Am 25: 655-678, viii. [Crossref]
- Roozenbeek B, Maas AI, Menon DK (2013) Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 9: 231-236. [Crossref]
- Langlois JA, Rutland-Brown W, Wald MM (2006) The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21: 375-378. [Crossref]
- Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99: 4-9. [Crossref]
- Kim JJ, Gean AD (2011) Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics 8: 39-53. [Crossref]
- Weiner LP (2008) Definitions and criteria for stem cells. Methods Mol Biol 438: 3-8. [Crossref]
- Galindo LT, Filippo TRM, Semedo P, Ariza CB, Moreira CM, et al. (2011) Mesenchymal Stem Cell Therapy Modulates the Inflammatory Response in Experimental Traumatic Brain Injury. Neurology Research International. 564089
- Harting MT, Sloan LE, Jimenez F, Baumgartner J, Cox CS Jr (2009) Subacute neural stem cell therapy for traumatic brain injury. J Surg Res 153: 188-194. [Crossref]
- Mahmood A, Lu D, Lu M, Chopp M (2003) Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurg 53: 693-702.
- World Medical Association. (2013) World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 310(20): 2191-2194.
- Ghajar J (2000) Traumatic brain injury. Lancet 356: 923-929. [Crossref]
- McKintosh T (2009) Novel Pharmacologic Therapies in the Treatment of Experimental Traumatic Brain Injury: A Review. Journal of Neurotrauma 10(3): 215-261.
- Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, et al.(2012) Administration of autologous bone marrow derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplantation 21 Supp 1: S1-S12.
- Sharma A, Sane H, Badhe P, Kulkarni P (2012) Autologous Bone Marrow Stem Cell Therapy shows functional improvement in hemorrhagic stroke- a case study. Indian Journal of Clinical Practice 23(2): 100-105
- Sharma A, Badhe P, Gokulchandran N, Chopra G (2012) Autologous bone marrow derived mononuclear cell therapy for vascular dementia - Case report. Journal of stem cell research and therapy. J Stem Cell Res Ther 2: 129.
- Sharma A, Gokulchandran N, Sane H, Badhe P, Kulkarni P, et al. (2013) Detailed analysis of the clinical effects of cell therapy for thoracolumbar spinal cord injury: an original study. Journal of Neurorestoratology 1: 13-22.
- Sharma A, Sane H, Paranjape A, Gokulchandran N, Nagrajan A, et al. (2015) The effect of autologous bone marrow mononuclear cell transplantation on the survival duration in Amyotrophic Lateral Sclerosis - a retrospective controlled study. Am J Stem Cells 4(1): 50-65.
- Zemlan F, Rosenberg W, Luebbe P (1999) Quantification of Axonal Injury in Traumatic Brain Injury: Affinity Purifaction and Characterization of Cerebrospinal Fluid Tau Proteins. Journal of Neurochemistry 72: 2.
- Cox CS Jr, Baumgartner JE, Harting MT, Worth LL, Walker PA, et al. (2011) Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery 68: 588-600. [Crossref]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, et al. (2000) Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164: 247-256.
- Zhong C, Qin Z, Zhong CJ, Wang Y, Shen XY (2003) Neuroprotective effects of bone marrow stromal cells on rat organotypic hippocampal slice culture model of cerebral ischemia. Neurosci Lett 342: 93-96
- Strauer BE, Kornowski R (2003) Stem cell therapy in perspective. Circulation 107: 929-934. [Crossref]
- Sharma A, Badhe P, Shetty O, Vijaygopal P, Gokulchandran N, et al. (2011) Autologous Bone Marrow Derived Stem Cells For Motor Neuron Disease with Anterior Horn Cell Involvement. Bombay Hospital Journal 53(1): 71-75.
- Liao G, Harting M, Hetz R, Walker PA, DO SKS, et al. (2015) Autologous Bone Marrow Mononuclear Cells Reduce Therapeutic Intensity for Severe Traumatic Brain Injury in Children. Pediatr Crit Care Med 16(3): 245-255.
- Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, et al. (2009) Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 18: 683-692. [Crossref]
- Sharma A, Sane H, Kulkarni P, Yadav J, Gokulchandran N, et al. (2015) Cell therapy attempted as a novel approach for chronic traumatic brain injury - a pilot study. Springerplus 4: 26. [Crossref]
- Kato T, Nakayama N, Yasokawa Y, Okumura A, Shinoda J, Iwama T. (2007) Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J Neurotrauma 24(6): 919-26.
- Nakashima T, Nakayama N, Miwa K, Okumura A, Soeda A, et al. (2007) Focal brain glucose hypometabolism in patients with neuropsychologic deficits after diffuse axonal injury. AJNR Am J Neuroradiol 28(2): 236-42.

