Abstract
Objective: This work is a retrospective review of the electrocorticography of 11 patients with intractable epilepsy who previously were implanted with a Responsive Neurostimulator (RNS, Neuropace™) who received any of the 3 clinically available SARS-CoV-2 vaccine series in the United States during the Covid-19 pandemic. The available vaccine products studied included the manufacturers Pfizer, Moderna, and Johnson & Johnson that were FDA approved to provide immunity against Covid-19.
Methods: After gaining institutional IRB approval, we performed a retrospective review of our RNS database and identified patients who received the initial series (without boosters) of SARS-CoV-2 vaccines after the World Health Organization declared the pandemic’s start date of March 11, 2020. We quantified the inventories of ictal and interictal discharges including short and long episode counts, channel saturations, magnet-swipes, and histogram data counts as arbitrary potentially surrogate correlates of seizure activity as other articles denote. Statistical analyses were conducted using R version 4.1.1 and tables were produced using the package gtsummary. Wilcoxon rank sum test was used for comparison of groups. A p-value of < 0.05 was considered significant.
Results: Although visual inspection of the electrocorticograms may suggest there are no significant changes overall in seizure activity, there was a variable clinical response to SARS-CoV-2 reflected in patients having statistically significant increases or decreases in total electrographic seizure activity and or long episodes. What substrate underlies this biological heterogeneity is unknown at this time.
Significance: Despite the small number of patients identified and confounders may not be excluded, RNS is a valuable method of studying patients post vaccination. To our knowledge, this is the first report indicating that there may be subtle biological variability of individual responses to SARS-CoV-2 vaccines on electrocorticography potentially due to unknown underlying heterogeneous inflammatory or other yet to be determined biological mechanisms.
Key words
intractable seizures, SARS-CoV-2, Responsive neurostimulation, Vaccines
Key Points
- RNS is a valuable method of studying the effects of vaccines on the electrocorticograms of patients who undergo vaccination series against SARS-CoV-2
- RNS identifies that subclinical seizure activity (Long Episodes- LEs) is increased in some patients who received vaccines for SARS-CoV-2 while such metrics may be decreased in other patients. The significance of the observations of this study as well as the mechanisms accounting for such biological heterogeneity are currently unknown.
- Further studies of the SARS-CoV-2 vaccine population using quantification that RNS allows may yield future valuable observations
Introduction
This work resulted from the request of several of our intractable epilepsy patients who were concerned about the safety of commercially available FDA approved vaccines against SARS-CoV-2 in the United States as they specifically feared the emergence of seizures or other side effects due to such vaccines. Some patients were concerned that even if the products themselves did not cause seizures directly, that if they experienced for example a febrile reaction after any vaccine, they could be at risk of febrile seizures that might be predicted from reviewing potentially identifiable subclinical changes in electrocorticography after receiving at least one or the initial dose within a vaccine series. Several patients with intractable epilepsy and previously implanted RNS devices implored that our Epilepsy team should monitor their electrocorticography either prospectively or retrospectively at least after an initial vaccine dose so they could make decisions regarding receiving any additional doses or products, or whether or not they should pre-medicate with a benzodiazepine such as lorazepam- although there currently is no scientific guidance regarding such clinical reasoning and associated sentiments in the currently available literature. It remains unknown what features, findings, or effects on electrocorticography could or should have impact on clinical decision making. This work sought therefore to determine if RNS devices might have utility in detecting changes on electrocorticography consistent with changes in seizure activity within a group of intractable epilepsy patients previously implanted with such devices associated with SARS-CoV-2 “Covid-19” Vaccination to guide clinical decision making.
Methods
After obtaining institutional IRB approval, we performed a retrospective review of our RNS database and identified patients within the patient data management system (PDMS) who had received doses of the 3 commercially available FDA vaccine products for SARS-CoV-2 “Covid-19” in the United States after March 11, 2020, which was designated the start of the Covid-19 pandemic by the World Health Organization (WHO) [1]. We quantified the daily inventories of ictal and interictal discharges, short and long episode counts, channel saturations, magnet-swipes, and thus the daily histogram data counts as other articles utilizing RNS as a method of monitoring and quantifying such information identify that such inventory may represent surrogate markers of seizure activity [2,3,4]. Statistical analyses were conducted using R version 4.1.1 and tables were produced using the package gtsummary [5]. Wilcoxon rank sum test was used for comparison of groups. A p-value of < 0.05 was considered significant.
Of note, 10 such patients completed their vaccination series with suitable data sets for analyses among the groups of Vaccines available that included products from Pfizer, Moderna, and Johnson & Johnson.
Results
Visual inspection of the electrocorticograms may not suggest any significant changes during and post vaccination series, and patient reporting of seizures and impression of changes during and post vaccination did not suggest any definite changes in seizure activity- however statistical analyses of the electrocorticogram dataset identifies that certain patients had either increases or decrease in total daily seizure activity and LEs- (LONG EPISODES) that met statistical significance at various time points post vaccination (Table 1, Figures 1&2). Although such results are noted sub clinically, we acknowledge that at this time they may be of limited or of unknown clinical significance at this time.
Table 1. Characteristics of patients under study. 5 Patients received the Moderna Vaccine series, 4 patients received the Pfizer vaccine series, and 2 patients received the Johnson & Johnson Vaccine series. Note age when implanted with RNS, current age, location of RNS stimulation, time since implantation as noted. (A) Left Bottom panel- results from statistical analysis of patients activities 4 weeks after the first vaccine dose compared to a baseline considered as such 4 weeks previously. (B) Right Bottom Panel- results from statistical analysis comparing activities 6 months after vaccine series completion compared with the noted baseline. the inventories of ictal and interictal discharges including short and long episode counts, channel saturations, magnet-swipes, and histogram data counts as arbitrary potentially surrogate correlates of seizure activity as other articles denote [2-4]. Statistical analyses were conducted using R version 4.1.1 and tables were produced using the package gtsummary [5]. Wilcoxon rank sum test was used for comparison of groups. A p-value of <0.05 was considered significant.
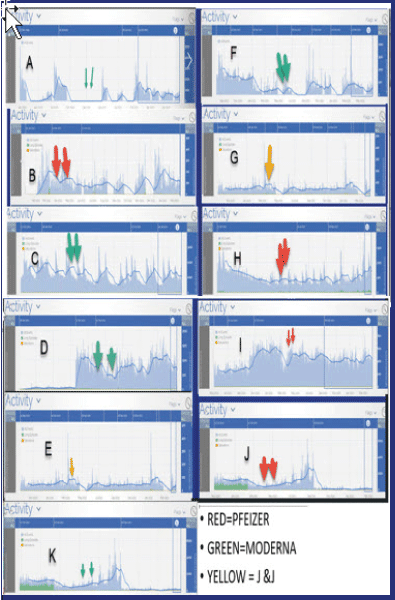
Figure 1: Histogram data identifying total seizure activity in relation to when vaccines were administered. Note that device adjustments, medication adjustments, and other confounders as noted in the discussion are not identified or excluded from the study or tabulation
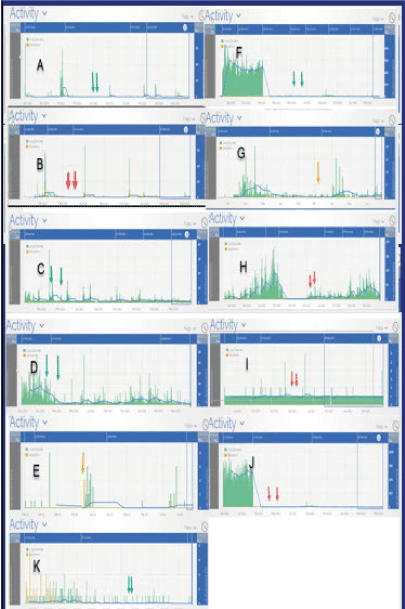
Figure 2: Histogram data identifying Long Episodes (LEs) and saturation events. As in table A, note that confounding factors delineated from the discussion are note excluded
Discussion
Articles outlining the initial experiences of patients and providers regarding the vaccination of patients for SARS-CoV-2 are noted, along with potential side effects of vaccines in general [6-10]. Vaccines have an association with seizures and febrile seizures especially in patients with pre-existing neurologic diagnoses [11-14]. The possible association of Hepatitis B vaccines with developing Multiple Sclerosis, and other papers denoting a possible association between the MMR(Measles-Mumps-Rubella) vaccine and autism which associations were all subsequently discredited are noted and as a consequence of reports such as those- the general public harbors some fear regarding the consequences of receiving vaccines [13]. Vaccines have been associated with developing Guillian -Barre’ Syndrome [15] Patients who may have an SCN1A gene mutation are noted to be at greater risk for vaccine encephalopathy after the pertussis vaccine, and the diphtheria tetanus and acellular vaccine (TDaP), inactivated polio (IPV), and Haemophilus type b (Hib) vaccines are associated with increased risks of febrile seizures in children [12-14]. The MMR vaccine is associated with an increased risk of febrile seizures 8 to 14 days after vaccination and there is a 5.7-fold increase in febrile seizures on the same day as the DPT vaccination noted has been along with a 2-3 fold increase in the two weeks following the first dose of the MMR vaccination [12-14].
The safety of the SARS-CoV-2 vaccination series has however so far been well established although articles such an association with vaccine induced Guillian-Barre Syndrome and myocarditis are noted -but the presence of seizure disorders has not otherwise been considered an absolute contraindication for any vaccine series for SARS-CoV-2 [15,16,17]. We acknowledge that the findings of this study may be quite limited due to the analysis of a small series of patients. We acknowledge that these findings reported here also may have limited if any clinical significance, and significant confounding may not be excluded. Such confounding influences include but are not limited to the effects of poor sleep, psychosocial stressors, device adjustments, medication changes, cyclical ultradian variation in seizure patterns, and other concomitant diagnoses or conditions that might have effects on seizures [2,3,18]. We are unable to determine why in some patients subclinical seizure activity as quantified and LE’s are increased and in some other patients such metrics are decreased and these findings will require further study. To our knowledge however – this is the first case series where RNS was used to study the effects of electrocorticography after vaccination.
References
- https://www.who.int [Accessed 22 June 2022].
- Leguia MG, Andrzejak RG, Rummel C, Fan JM, Mirro EA, et al. (2021) Seizure Cycles in Focal Epilepsy. JAMA Neurol 78: 454-463. [Crossref]
- Oster JM, Tatum P, Monigan C, Kryzanski J (2022) Seizures Noted by Responsive Neurostimulation From e-Cigarette Use (Vaping). J Clin Neurophysiol 39: 1-3. [Crossref]
- Karakas C, Ward R, Hegazy M, Skrehot H, Haneef Z (2022) Seizure control during the COVID-19 pandemic: Correlating Responsive Neurostimulation System data with patient reports. Clin Neurophysiol 139: 106-113. [Crossref]
- Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J (2021) Reproducible Summary Tables with the gtsummary Package. The R Journal 13: 570-580.
- Kuroda N (2021) Epilepsy and COVID-19: Updated evidence and narrative review. Epilepsy Behav 116: 107785. [Crossref]
- von Wrede R, Pukropski J, Moskau-Hartmann S, Surges R, Baumgartner T (2021) COVID-19 vaccination in patients with epilepsy: First experiences in a German tertiary epilepsy center. Epilepsy Behav 122: 108160. [Crossref]
- Lu L, Xiong W, Mu J, Zhang Q, Zhang H, et al. (2021) The potential neurological effect of the COVID-19 vaccines: A review. Acta Neurol Scand 144: 3-12. [Crossref]
- Lu L, Zhang Q, Xiao J, Zhang Y, Peng W, et al. (2022) COVID-19 vaccine take-up rate and safety in adults with epilepsy: Data from a multicenter study in China. Epilepsia 63: 244-251. [Crossref]
- Scheffer IE (2015) Vaccination Triggers, Rather Than Causes, Seizures. Epilepsy Curr 15: 335-337. [Crossref]
- Sun Y, Christensen J, Hviid A, Li J, Vedsted P, et al. (2012) Risk of febrile seizures and epilepsy after vaccination with diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and Haemophilus influenzae type B. JAMA 307: 823-831. [Crossref]
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, et al. (2013) Vaccine hesitancy: an overview. Hum Vaccin Immunother 9: 1763-1773. [Crossref]
- Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, et al. (2001) The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med 345: 656-661. [Crossref]
- Atzenhoffer M, Auffret M, Pegat A, Masmoudi K, Khouri C, et al. (2022) Guillain-Barré Syndrome Associated with COVID-19 Vaccines: A Perspective From Spontaneous Report Data. Clin Drug Investig 42: 581-592. [Crossref]
- Salah HM, Mehta JL (2021) COVID-19 Vaccine and Myocarditis. Am J Cardiol 157: 146-148. [Crossref]
- CDC (2019) Safety of COVID-19 Vaccines. Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/safety-of-vaccines.html [Accessed 27 July 2022]
- Huang S, Wu C, Jia Y, Li G, Zhu Z, et al. (2020) COVID-19 outbreak: The impact of stress on seizures in patients with epilepsy. Epilepsia 61: 1884-1893. [Crossref]
- Malow BA (2004) Sleep deprivation and epilepsy. Epilepsy Curr 4: 193-195. [Crossref]


