Abstract
Endogenous digoxin-like factors (EDLF) are compounds that promote natriuresis, inhibit sodium-potasium-ATPase, and cross-react with antibodies raised against cardiac glycosides. Although EDLF are well described in experimental animal models and a variety of clinical conditions in humans, no investigation previously evaluated an association between EDLF and hypothermia. We used two separate models to examine the presence of EDLF in hypothermic humans.
The first study consisted of a case-controlled comparison of patients with unintentional environmental hypothermia. All patients with environmental hypothermia were prospectively enrolled if they were not taking cardiac glycosides and had no conditions previously associated with EDLF. A digoxin concentration obtained at presentation was compared to one obtained from presentation on patients from a demographically matched control group of normothermic patients. The second study enrolled a convenience sample of patients undergoing controlled cold cardioplegia for surgery. Once again, patients were excluded for conditions previously associated with EDLF, and were required to have a non-detectable preoperative digoxin concentration. Digoxin concentrations were subsequently compared in the pre- and post-operative periods. In addition to serving as their own controls, a comparison group of normothermic patients were added to help control for confounding factors associated with surgery under general anesthesia.
Twenty-two patients with environmental hypothermia (mean temperature 91° Fahrenheit, range, 83.8-93.8° Fahrenheit) and 22 controls were included in the first study. Ten hypothermic patients had measurable digoxin concentrations, 6 of which were above 0.15 ng/mL. All control patients had digoxin concentrations of 0.00 ng/mL (p = 0.03, Fisher's exact test). The second study enrolled ten patients undergoing surgery with cold cardioplegia and ten control patients undergoing normothermic surgery under general anesthesia. None of the ten control patients had measurable digoxin concentrations on either pre or postoperative testing. Although all of the cold cardioplegia hypothermic patients had negative preoperative digoxin concentrations, one developed a postoperative concentration of 0.44 ng/mL. We conclude that hypothermia is associated with the presence of EDLF. An animal model and further human testing will be required to establish causation.
Introduction
In 1976, Besch noted falsely elevated digoxin concentrations in premature infants who were not exposed to cardiac glycosides [1]. It is now well recognized that tissues from humans and a variety of animal species contain material that inhibits sodium-potasium-ATPase, stimulates natruresis, and is cross-reactive with antibodies raised against digoxin and other cardiac glycosides. Although originally termed endogenous digitalis-like binding substance (EDLS), the recognition that multiple compounds are involved has given rise to the term endogenous digoxin-like factors (EDLF). These endogenous digoxin-like factors can be induced by salt-loading in animals [2,3]. In addition, EDLF can be found in humans with myocardial infarction [4,5], renal failure [6-8], congestive heart failure [9,10], hepatic failure [11], and subarachnoid hemorrhage [12]. Moreover, human placenta [13,14], is a known source of DLF, and EDLF are found in association with both normal pregnancy [15], and pre-eclampsia [16,17], as well as in newborns [18]. Finally, EDLF are also described in association with hypoglycemia [19] and strenuous exercise [20]. All of these conditions share marked abnormalities of sodium balance or intravascular fluid status [2,21].
At least one of the EDLF is, in all likelihood, a bufodienolide, distinguishing it from most plant-derived cardiac glycosides, which are cardenolides (Figure 1). This endogenous steroid is thought to be produced in the adrenal cortex [22,23] and its function is essentially indistinguishable from pharmacologically administered cardiac glycosides.
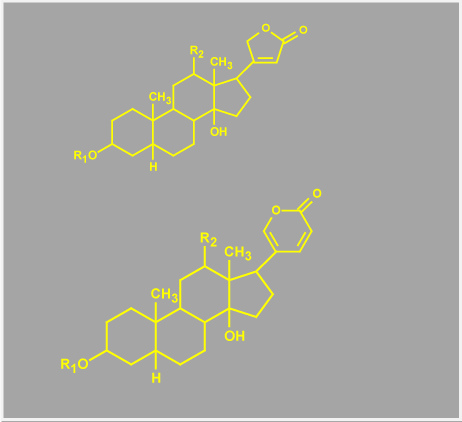
Figure 1. Chemical comparison of cardenolides and bufodenolides. The figure shows the two basic structures of cardioactive steroids, the upper image being a cardenolide defined by the 5 membered lactone ring and the lower image defines a bufodenolide with its 6 membered lactone ring.
Recently, we cared for a patient with environmental hypothermia who maintained a profound bradycardia, which persisted for a few hours even after rewarming. Although he denied ingestion of plant or herbal products and was on no cardiac medications, his digoxin concentration was positive. As a result, we designed the following two-part study to systematically investigate the association between EDLF and hypothermia.
Methods
Both study protocols where individually reviewed and approved by the institutional review boards of New York University Medical Center, Bellevue Hospital Center and the New York City Health and Hospital’s Corporation. All digoxin concentrations were measured on waste blood from routine phlebotomy using the Boehringer-Manhiemer radio-immuno assay, which was the standard digoxin assay used at both institutions during the study period [24-26]. Digoxin concentrations in patients not exposed to cardiac glycosides or with no known risk factors for ELDF are routinely recorded as 0.00 ng/mL using this technique, although the laboratory reported values less than 0.15 ng/mL as negative for clinical purposes.
Environmental hypothermia
Inclusion criterion: Using a prospective and consecutive study design, all adult patients presenting to the emergency department with a core rectal temperature less than 95°F were eligible for study.
Exclusion criteria: Patients who were taking cardiac glycosides by history or were suspected of having a diagnosis associated with elevated EDLF (as mentioned previously) were excluded from the study.
Evaluation: Basic demographic data, a medical history and a physical examination were recorded. Patients were evaluated with a chest radiograph, ECG and standard laboratory tests at the discretion of the treating physician. Additionally, all participants had a serum digoxin concentration that was obtained from their initial phlebotomy. Since historical data at our institution suggested a high association between alcohol intoxication and environmental hypothermia, a control group was obtained to help exclude alcohol as a confounding variable. Therefore, digoxin concentrations were also measured in a convenience sample of demographically matched normothermic patients with elevated blood alcohol concentrations.
Data analysis: Demographic data were compared with a Chi Square test, Fisher's exact test, or Student's T-test as appropriate. The number of patients with measurable digoxin concentrations in the environmental hypothermia and control groups was compared using a Fisher’s exact test. A p<0.05 was considered statistically significant. Data were analyzed using the EPISTAT statistical package (Richardson, Texas).
Controlled hypothermia
Inclusion criterion: All patients undergoing cold cardioplegia for cardiac surgery were eligible for enrollment. A convenience sample was selected based on investigator availability.
Exclusion criteria: Patients were excluded if they had known congestive heart failure, were already taking digoxin or another cardiac glycoside or had a co-morbid condition previously associated with EDLF.
Evaluation: A pre-operative digoxin concentration was measured within four hours of surgery. By operative protocol, all patients were cooled to a core body temperature between 87.8° and 89.6°F. During surgery, an intra-operative digoxin concentration was measured at the nadir of body temperature. Post-operative digoxin concentrations were measured within four hours of returning to normothermia (defined as a core temperature greater than 95°F). The operative record including the type of surgery, duration of hypothermia, intra-operative medications and cardiac bypass time was abstracted on a standardized data collection instrument. Although each subject served as his or her own control, an additional control group of demographically matched patients undergoing general anesthesia, without a period of controlled hypothermia, was identified to help exclude possible confounding effects of general anesthesia and surgery. Digoxin concentrations were only measured in the preoperative and immediate post-operative period in these patients.
Data analysis: The digoxin concentrations in the controlled hypothermia patients were compared to baseline with a paired Student's T-test. The number of patients with measurable digoxin concentrations in the controlled hypothermia and normothermic general surgery groups was compared using a Fisher’s exact test, with a p<0.05 considered statistically significant. Once again data were analyzed using the EPISTAT statistical package (Richardson, Texas).
Results
Twenty-two hypothermic emergency department patients were evaluated for EDLF. Their mean age was 57 years, and twenty were male. Their mean core body temperature on presentation was 91°F (range, 83.8°-93.8°F). Their mean blood alcohol concentration was 318 mg/dL (range 195-625 mg/dL). All patients were successfully rewarmed with passive external techniques as previously described [27]. The emergency department control group were not statistically different with regard to demographic data and blood alcohol concentrations as shown in Table 1.
Parameter |
Hypothermic |
Controls |
Mean Age (range) |
56 (35-93) |
52 (30-66) |
Male/Female |
20/3 |
20/3 |
Mean Temperature oF (range) |
90.6 (83.8-94.6) |
98.8 (96.8-101.2) |
Mean Ethanol Concentration in mg/dL (range) |
284 (<10-625) |
322 (150-599) |
Median Bedside Glucose in mg/dL (range) |
97 (71-566) |
88 (48-266) |
Mean Blood Urea Nitrogen (range) |
14 (6.7-28) |
8 (5-14) |
Mean Digoxin Concentration in ng/mL (range) |
0.1 (0-0.4) |
All <0.15 |
Number With Digoxin ≥ 0.15 ng/ml |
7 |
0 |
Table 1. Demographic data and blood alcohol concentrations.
Patient # |
Age |
M/F |
Temp
(°F) |
Ethanol
(mg/dL) |
Glucose
(mg/dL) |
BUN
(mg/dL) |
Digoxin
(ng/mL) |
1 |
75 |
M |
93.8 |
254 |
75 |
24.5 |
0.4 |
6 |
60 |
M |
85.8 |
298 |
81 |
7.3 |
0.2 |
7 |
56 |
M |
90.8 |
625 |
105 |
6.9 |
0.2 |
13 |
53 |
M |
92.6 |
211 |
85 |
8.6 |
0.4 |
14 |
63 |
M |
83.8 |
408 |
141 |
6.7 |
0.2 |
23 |
44 |
F |
94 |
195 |
84 |
8.9 |
0.3 |
24 |
53 |
M |
92.1 |
55 |
117 |
11.5 |
0.23 |
2021 Copyright OAT. All rights reserv
Table 2. Characteristics of the 7 patients with environmental hypothermia and elevated digoxin concentrations
At the time of presentation, none of the normothermic control group had a positive digoxin assay. In ten hypothermic patients, a positive digoxin assay was recorded. Four hypothermic patients had digoxin concentrations of 0.1 ng/mL. As stated previously, although the laboratory reported digoxin concentrations less than 0.15 ng/mL as negative with regard to clinical relevance, patients who are not taking digoxin usually have concentrations of 0.00 ng/mL. Regardless of that fact, these four patients were excluded from further analysis because of possible ambiguity. Six patients had digoxin concentrations greater than 0.2 ng/mL (mean digoxin concentration 0.27 ng/mL, range 0.2-0.4 ng/mL), and were included in the final data analysis. Thus, patients with unintentional environmental hypothermia were significantly more likely to have a positive digoxin concentration than controls (p=0.03, Fisher's Exact test).
Ten patients who had controlled hypothermia during cardiac bypass were enrolled. Their mean age was 53 years, and seven were male. Six patients underwent cardiac bypass for coronary artery bypass grafting, while the remaining patients underwent cardiac bypass for valve replacement (1 mitral and 3 aortic). Their mean duration of cardiac bypass (pump time) was 2.1 hours and all patients were hypothermic for at least one hour. Preoperative digoxin concentrations were 0.00 ng/mL in all ten patients. One patient subsequently developed a positive digoxin concentration at 0.44 ng/mL. The normothermic general surgery patients consisted of 6 male and 4 female patients with a mean age of 49 years old. All patients underwent laparotomy for bowel (8) or gallbladder (2) surgery using combined inhalational and intravenous anesthetic techniques. Both preoperative and postoperative digoxin concentrations were 0.00 ng/mL in every patient. Although not statistically different than the control group, the finding of one patient who developed EDLF in the controlled hypothermia group may have clinical relevance.
Discussion
Since their first description 25 years ago, significant advances have been made in the understanding of EDLF. It is now generally accepted that the physiologic roles of EDLF include; promotion of naturesis, enhancing vasoconstriction by inhibition of the Na+-K+ pump in vascular smooth muscle, and increasing cardiac inotropy by the inhibition of Na+-K+-ATPase [3,28]. Additionally, like other cardiac glycosides, EDLF are now recognized as prodysrhythmic agents [29-31]. Finally, the many similarities between EDLF and digoxin are further supported by the reversal of the effects of EDLF by polyclonal anti-digoxin antibodies [30-32].
We hypothesized that physiologic stresses associated with hypothermia might be sufficient to provoke the release of EDLF. Our finding of elevated digoxin concentrations in hypothermic patents suggests and association between hypothermia and EDLF. To the best of our knowledge, this is the first description of this association.
Several limitations of this study require discussion. With regard to the digoxin assay, we recognise that an unexpected positive digoxin concentration is only a surrogate for EDLF, as no direct assay exists. Although unlikely, other cross-reacting substances could have resulted the positive digoxin assays found in these patients. Also, for the purposes of this study, we used the standard digoxin assay that was available in our clinical hospital laboratory at the time of the investigation. Although this polyclonal assay was expressly calibrated for digoxin, we have previously demonstrated its cross-reactivity with other cardioactive steroids, and specifically, bufodenolides [33-35]. In fact, this problem may have increased the false-negative rate in our study patients, as a low concentration of cross-reactivity would have been interpreted as a negative result as was done with the 4 patients in the unintentional hypothermia group with measurable concentrations that were less than 0.15 ng/mL. It is of note that if such an effect were if present, it would only strengthen the association described herein.
Another consideration must relate to one of causation. Urban patients with unintentional hypothermia and alcoholism comprise a unique subset of hypothermic patients with complex medical problems. Although our use of matched control patients should have eliminated many confounding variables that could have been responsible for the presence of EDLF it must be noted that an unrecognised factor not related to hypothermia may have been responsible for the EDLF in these patients. Additionally, although it was desirable, the non-interventional nature of the first study protocol prohibited sequential phlebotomy to confirm the disappearance of EDLF with rewarming. Unfortunately, only one patient in controlled hypothermia study developed EDLF. Although this was not statistically significant, it offers support for our earlier findings, as we were unable to find EDLF in normothermicgeneral surgery patients. The small size of the controlled study, however, may have introduced a type II statistical error into the results.
Finally, the contrast between the controlled hypothermia and unintentional hypothermia patients requires discussion. If hypothermia is in fact a trigger for EDLF, then we would speculate that either the short duration of operative hypothermia or the hemodynamic support offered by cardiac bypass and the controlled anaesthesia environment might provide an insufficient stress, even in the setting of hypothermia, to trigger the formation of EDLF in most patients. Alternatively, the significant hemodilution that occurs during bypass surgery may have dropped the concentration of EDLF below the detection threshold. In the case of the one patient who developed EDLF, we were unable to identify any event that was different than other patients in the controlled hypothermia study.
We conclude that there is an association between hypothermia and EDLF. It is unclear whether hemodynamic changes, or either sodium or fluid balance contributes to this finding. Thus, further study is required to clarify the nature of this association. Specifically, a controlled animal model may help exclude some of the confounding variables that are present in many clinical studies. Additionally, a possible role should be explored for the use of anti-digoxin antibodies in the treatment of hypothermia-associated dysrhythmias.
References
- Besch HR, Hufferd S, Lake M, Hurwita R, Watanabe AM (1976) False elevations of apparent digoxin levels in plasma of premature infants. Clin Chem 22: 1168.
- Gruber KA, Whitaker JM, Buckalew VM Jr (1980) Endogenous digitalis-like substance in plasma of volume-expanded dogs. Nature 287: 743-745. [Crossref]
- Bagrov AY, Fedorova OV, Dmitrieva RI, French AW, Anderson DE (1996) Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anesthetized dogs. Cardiovasc Res 31: 296-305. [Crossref]
- Bagrov AY, Kuznetsova EA, Fedorova OV (1994) Endogenous digoxin-like factor in acute myocardial infarction. J Intern Med 235: 63-67. [Crossref]
- Bagrov AI, Kuznetsova EA, Fedorova OV (1996) [Endogenous digoxin-like factor in myocardial infarction]. Klin Med (Mosk) 74: 15-17. [Crossref]
- Craver JL, Valdes R Jr (1983) Anomalous serum digoxin concentrations in uremia. Ann Intern Med 98: 483-484. [Crossref]
- Isensee L, Solomon RJ, Weinberg MS, et al. (1988) Digoxin levels in dialysis patients. Hosp Physician 24: 50–52.
- Graves SW, Brown B, Valdes R Jr (1983) An endogenous digoxin-like substance in patients with renal impairment. Ann Intern Med 99: 604-608. [Crossref]
- Gottlieb SS, Rogowski AC, Weinberg M, Krichten CM, Hamilton BP, et al. (1992) Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation 86: 420-425. [Crossref]
- Shilo L, Adawi A, Solomon G, Shenkman L (1987) Endogenous digoxin-like immunoreactivity in congestive heart failure. Br Med J (Clin Res Ed) 295: 415-416. [Crossref]
- Nanji AA, Greenway DC (1985) Falsely raised plasma digoxin concentrations in liver disease. Br Med J (Clin Res Ed) 290: 432-433. [Crossref]
- Wijdicks EF, Vermeulen M, van Brummelen P, den Boer NC, van Gijn J (1987) Digoxin-like immunoreactive substance in patients with aneurysmal subarachnoid haemorrhage. Br Med J (Clin Res Ed) 294: 729-732. [Crossref]
- Paci A, Cocci F, Piras F, Ciarimboli G, Clerico A (1989) Specific binding of cardiac glycoside drugs and endogenous digitalis-like substance to particulate membrane fractions from human placenta. Clinical Chemistry 35: 2093-2097. [Crossref]
- Hilton PJ, White RW, Lord GA, Garner GV, Gordon DB, et al. (1996) An inhibitor of the sodium pump obtained from human placenta. Lancet 348: 303-305. [Crossref]
- Friedman HS, Abramowitz I, Nguyen T, Babb B, Stern M, et al. (1987) Urinary digoxin-like immunoreactive substance in pregnancy. Relation to urinary electrolytes. Am J Med 83: 261-264.[Crossref]
- Graves SW, Valdes R Jr, Brown BA, Knight AB, Craig HR (1984) Endogenous digoxin-immunoreactive substance in human pregnancies. J Clin Endocrinol Metab 58: 748-751. [Crossref]
- Koren G, Farine D, Grundmann H, Heyes J, Soldin S, et al. (1988) Endogenous digoxin-like substance(s) associated with uneventful and high-risk pregnancies. Dev Pharmacol Ther 11: 82-87.[Crossref]
- Valdes R Jr, Graves SW, Brown BA, Landt M (1983) Endogenous substance in newborn infants causing false positive digoxin measurements. J Pediatr 102: 947-950. [Crossref]
- Graves SW, Adler G, Stuenkel C, Sharma K, Brena A, et al. (1989) Increases in plasma digitalis-like factor activity during insulin-induced hypoglycemia. Neuroendocrinology 49: 586-591.[Crossref]
- Valdes R Jr, Hagberg JM, Vaughan TE, Lau BW, Seals DR, et al. (1988) Endogenous digoxin-like immunoreactivity in blood is increased during prolonged strenuous exercise. Life Sci 42: 103-110. [Crossref]
- Takahashi H, Matsusawa M, Okabayashi H, Suga K, Ikegaki I, et al. (1988) Endogenous digitalislike substance in an adult population in Japan. Am J Hypertens 1: 168S-172S. [Crossref]
- Flier JS, Maratos-Flier E, Pallotta JA, McIsaac D (1979) Endogenous digitalis-like activity in the plasma of the toad Bufo marinus. Nature 279: 341-343. [Crossref]
- Morris JF, Poston L, Wolfe CD, Hilton PJ (1988) A comparison of endogenous digoxin-like immunoreactivity and sodium transport inhibitory activity in umbilical arterial and venous serum. Clin Sci (Lond) 75: 577-579. [Crossref]
- Vinge E, Ekman R (1988) Evaluation of cross-reactivity of urinary digoxin-like substance in different radioimmunoassays. Ther Drug Monit 10: 316-320. [Crossref]
- Naomi S, Graves S, Lazarus M, Williams GH, Hollenberg NK (1991) Variation in apparent serum digitalis-like factor levels with different digoxin antibodies. The "immunochemical fingerprint". Am J Hypertens 4: 795-801. [Crossref]
- Goto A, Yamada K, Ishii M, Sugimoto T, Yoshioka M (1991) Immunoreactivity of endogenous digitalis-like factors. Biochem Pharmacol 41: 1261-1263. [Crossref]
- Vassallo SU, Delaney KA, Hoffman RS, Slater W, Goldfrank LR (1999) A prospective evaluation of the electrocardiographic manifestations of hypothermia. Acad Emerg Med 6: 1121-1126.[Crossref]
- Bagrov AY, Dmitrieva RI, Fedorova OV, et al. (1996) Endogenous marinobufagenin-like immunoreactive substance. A possible endogenous Na, K-ATPase inhibitor with vasoconstrictor activity. Am J Hypertension 9: 982-990. [Crossref]
- Bagrov AY, Fedorova OV, Roukoyatkina NI, Zhabko EP (1993) Effect of endogenous digoxin-like factor and digoxin antibody on myocardial Na+, K(+)-pump activity and ventricular arrhythmias in acute myocardial ischaemia in rats. Cardiovasc Res 27: 1045-1050. [Crossref]
- Bagrov AI, Kuznetsova EA, Fedorova OV (1996) [Endogenous digoxin-like factor in myocardial infarction]. Klin Med (Mosk) 74: 15-17. [Crossref]
- Bagrov AY, Veveris MM, Ganelina IE, et al. (1991) Anti-arrhythmia effect of digoxin antibodies in experimental myocardial infarct (arrhythmogenic action of endogenous digoxin-like factor)]. Biull Eksp Biol Med 112: 20-22. [Crossref]
- Bagrov AY, Fedorova OV, Austin-Lane JL, et al. (1995) Endogenous marinobufagenin-like immunoreactive factor and Na+, K+ ATPase inhibition during voluntary hypoventilation. Hypertension 26: 781-788. [Crossref]
- Bania T, Hoffman RS, Howland MA, Goldfrank LR (1993) Accidental Indian hemp (Apocyneacea cannabinum) cardiac glycoside toxicity. Vet Hum Toxicol 35: 328.
- Brubacher JR, Hoffman RS, Kile T (1996) Toad venom poisoning: failure of a monoclonal digoxin immunoassay to cross-react with the cardioactive steroids. J Toxicol Clin Toxicol 34: 529-530.[Crossref]
- Brubacher JR, Ravikumar PR, Bania T, Heller MB, Hoffman RS (1996) Treatment of toad venom poisoning with digoxin-specific Fab fragments. Chest 110: 1282-1288. [Crossref]

