Abstract
Myofascial trigger points (MTrPs) can be a significant source of pain. This manuscript reviews the literature on the numerous interventions used to abate the signs and symptoms of MTrPs. This includes spray and stretch, topical agents, injections, trigger point compression, ultrasound, electrical stimulation, laser, dry needling, taping, and instrument assisted soft tissue techniques. The manuscripts cited reveal the results of these interventions in isolation, as well as, in conjunction with other modalities.
Key words
trigger points, physical modalities, soft tissue pain
Myofascial trigger points (MTrPs) can be a significant source of pain. Up to 85% of people who present to pain clinics have MTrPs. The prevalence is greater in women than men and the frequency of locations are head/neck, shoulders, extremities, and low back, in that order. The etiology of MTrPs has been described as repetitive, overload or overuse of muscles, asymmetries, or biomechanical discrepancies [1].
MTrPs are defined as latent or active [2,3]. Latent MTrPs are far more common than active MTrPs. Latent MTrP may cause stiffness & restricted ROM. There may be a focus of hyperirritability in muscle or fascia that is clinically quiescent with respect to spontaneous pain and are painful only when palpated. Whereas, active MTrPs have a focus of hyperirritiability in a muscle or its fascia, that is symptomatic with respect to pain. Active MTrPs refer a pattern of pain at rest and/or on motion that is specific for the muscle [4,5]. This focus or “knot” is a histochemical milieu of eight substances found in greater concentration in MTrPs than in normal, pain-free muscle: Calcitonin gene-related peptide, Interleukin-1β, proton [H+] concentrations, bradykinin, substance P, tumor necrosis factor-α, serotonin, and norepinephrine [6]. The pathogenesis has been suggested to be acute, chronic or persistent overloading of a muscle. The activation of the actin-myosin contractile mechanism increases the metabolic rate which leads to an increase in metabolites, the firing of nociceptors, and local/referred pain. Sustained contractions decrease local blood flow which decreases ATP availability. The reduced ATP interferes with the effectiveness of the calcium pump leading to a vicious cycle. When active locus (spontaneous electrical activity), sensitive locus (local twitch response), and nociceptor (pain receptor) coincide, a MTrP is formed [7]. The oversimplified, conventional, and controversial wisdom has identified a trigger point as “an unholy clump of contracted sarcomeres living in a swamp of their own garbage molecules, waste metabolites” [8]. Thomas and Shankar [9] reported “A growing body of evidence that suggests that taut bands are readily visualized under ultrasound-guided exam, especially when results are correlated with elastography, multidimensional imaging, and physical exam findings such as local twitch response.”
The symptoms of MTrPs may include exquisite tenderness, “jump sign” (snapping with palpation), referred pain with sustained pressure (does not follow a dermatome or nerve distribution), increased skin temperature (1.5°C increase in active MTrP; 1.0°C increase in latent MTrP), and decreased skin impedance but there are no changes in lab values or EMG at rest [10-12]. Travell and Simon [10] have published extensive works on the MTrP referral patterns of every muscle of the body.
Interventions
Over many decades, a plethora of interventions have been explored to mitigate the signs and symptoms of MTrPs [13,14]. These include, but are not limited to, spray and stretch, topical agents, injections, trigger point (TrP) compression, ultrasound/phonophoresis, electrical stimulation, LASER, dry needling, taping, and instrument assistant soft tissue techniques (IASTT). Vernon and Schneider [15] reviewed 112 manuscripts and concluded there is strong evidence to support the use of laser, moderate evidence for electrical nerve stimulation, acupuncture, and magnet therapy, and weak evidence of electrical muscle stimulation, galvanic stimulation, interferential stimulation, and ultrasound. There have been numerous studies published since 2009. There are been reports of varying levels of success but many of the studies have failed to reveal detailed parameters for either clinical application or laboratory replication.
Spray and stretch
Spray and stretch techniques were one of the three methods published by Travel and Simon over 20 years ago. The method involved anchoring the target muscle, applying a passive stretch, and then performing parallel sweeps through the MTrP at a rate of four inches per second, at a 30° angle to the skin, approximately 12 inches away. Sweeps would overlap each other and the distance from the skin would be adjusted based on the desired “coldness,” i.e. if too cold, hold spray closer to skin; if not cold enough, hold spray further from skin [11,16]. Most clinicians believe treatments should be directed to inactivating the MTrP prior to stretching or strengthening the muscle [1]. The simplified image below (Figure 1) compares the normal chain of sarcomeres with that of a chain of MTrP. If the sarcomeres of the MTrP are clumped together, then attempting to stretch a muscle will result in the over-stretching of the adjacent sarcomeres.
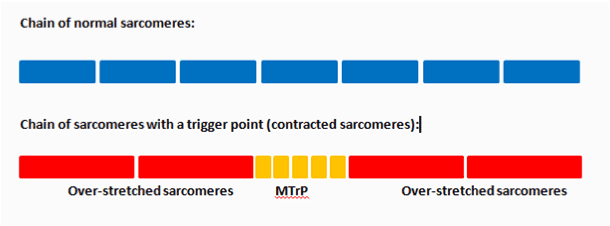
Figure 1. Normal sarcomere versus sarcomeres of a trigger point.
Topical agents.
The releasing of the MTrP has been attempted via a multitude of substances. Topical agents such as thiocolchicoside (Muscoril), Lidocaine, Diclofenac, and many over-the-counter substances have been studies. Efficacy of topical thiocolchicoside (Muscoril) on acute cervical MTrP was studied by Ketenci et al. [17]. Three groups (n=65) of individuals with MTrP were given a topical, injection, or both treatments over five consecutive days. Outcome measures were pain pressure threshold (PPT), range of motion (ROM), and visual analogue scale (VAS) for pain. Topical and injection groups had an improved VAS after the first treatment, whereas the combined treatment took three treatments to improve VAS. There was no significant difference in PPT or ROM.
Effectiveness of a heated Lidocaine/Tetracaine topical patch for pain associated with MTrPs was examined by Rauck et al. [18]. Twenty patients enrolled and 17 completed the study. One patch was applied to a MTP for four hours, two times per day for two weeks. At baseline, average pain intensity was 6.3 ± 1.56. Pain decreased by 33% to 4.5 ± 2.31 at the end of treatment. Forty percent of the participants had a clinically significant (≥ 30%) decrease, and 25% had a substantial (≥ 50%) decrease in pain. This pain reduction enabled more than 35% of the participants to increase their general activity, mood, normal work, and enjoyment of life and more than 50% of the participants to improve walking, sleep, and relationships. Two weeks after stopping treatment, average pain intensity was 5.0 ± 2.04 and treatment benefit was maintained in eight (40%) participants. At the conclusion, 75% were satisfied or very satisfied with treatment and none required rescue medication. Thus despite an adverse event of erythema, the heated lidocaine/tetracaine patch was reported to have potential utility as a noninvasive pharmacologic approach for managing MTrP pain.
A randomized, controlled study comparing a lidocaine patch, a placebo patch, and an anesthetic injection for treatment of TrPs was performed by Affaitati et al. [19]. Sixty individuals with MTrPs were randomly assigned to one of three groups: Lidocaine patch (replaced every 12 hours x 4 days), placebo patch (4 days), or two (1-ml) injections of bupivacaine hydrochloride (given 2 days apart). The results were a significant decrease in pain with the lidocaine patch and the injection.
Likewise, Hsieh et al. [20] studied the efficacy and side effects of diclofenac patches in treatment of patients with myofascial pain syndrome of the upper trapezius. Participants (n=153) were divided into two groups: diclofenac patch or control (menthol patch). The patch was 10 x 14 cm and was applied three times per week for one week. Outcome measures were VAS, cervical ROM, PPT, and Neck Disability Index (NDI). The diclofenac patch was statistically superior to the control for all time intervals for VAS, ROM, and NDI but no different for PPT. Table 1 displays the result of this study. At the end of the study, 20 people in the diclofenac patch group reported the tolerability of the treatment to be “very good.”
Table 1. Diclofenac Patch Outcomes [20]
Diclofenac Patch Outcomes |
|
Time Change |
Treatment (%) |
Control (%) |
VAS for pain |
Day 4 to Day 0
Day 8 to Day 0
Day 8 to Day 4 |
-26.90
-51.33
-33.42 |
-21.21
-25.76
-5.77 |
Cervical ROM |
Day 4 to Day 0
Day 8 to Day 0
Day 8 to Day 4 |
8.54
18.38
16.93 |
-1.70
6.61
8.46 |
Neck Disability |
Day 4 to Day 0
Day 8 to Day 0 |
-20.63
-32.35 |
-16.85
-25.65 |
Another study by Avrahami et al. [21] explored the effect of five over-the-counter analgesics of myofascial pain. One-hundred, twenty people were randomly assigned to one of six groups: 1) Professional Therapy MuscleCare Roll-on, 2) Motion Medicine cream, 3) Bengay Ultra Strength, 4) Icy Hot Extra Strength, 5) Biofreeze roll-on gel, and 6) control. Each group received a single application of the topical analgesic. PPT was tested before and 5-minutes after the application. MuscleCare (p=0.0002), Motion Medicine (p=0.01), and Ben-Gay (p=0.002) all increased the PPT. This was surprising there was a change in just 5-minutes after application. Results of testing 30 or 60 minutes after application would have been interesting.
Finally, Cho et al. [22] examined the efficacy of a 0.1% capsaicin hydrogel patch for myofascial neck pain. Sixty-one people were randomly assigned to one of two groups: patch with and patch without capsaicin. The patch was applied for 12 hours daily for four weeks. Outcome data included VAS, NDI, and Beck’s Depression Inventory (BDI) at baseline, after two weeks, and after four weeks of treatment. Although all improved, there was no significant difference between the groups.
Injections
Borg-Stein and Simons [23] stated MTrP injections should not be used as an isolated treatment. The pathogenesis of a MTrP injection have been theorized to result in a interruption of pain feedback mechanism, mechanical disruption of muscle fibers, vasodilatory effect to remove metabolites, and local dilution of nociceptive substances [2]. Typically a 22-gauge, 1.5” needle is used for superficial MTrPs and a 21-gauge, 2.5” needle for deep MTrPs. The syringe is advanced at a 30° angle and the use of ultrasound-guided technique may help confirm proper needle placement, i.e. in muscle and not in adipose. Baldry [24] reported keeping the needle in place for up to 30 seconds may terminate the dysfunctional activity of the motor end plate. A variety of medications can be utilized: 0.5% procaine hydrochloride, 3% promethazine-hydrochloride, 1% lidocaine hydrochloride, 0.25% lidocaine, and Botulinum toxin A, to name a few [7]. Lugo et al. [25] examined the influence of physical therapy (PT), lidocaine injection (LI), and the combination (PT+LI) on VAS, SF-36, and Patient Health Questionnaire (PHQ). Physical therapy consisted of 12 sessions (3x/wk x 4 wks) with 10 minutes of heat, 10 minutes of 1-MHz US at 0.8 W/cm2, MTrP deactivation with compression, and 20 minutes of exercise. Attendance of those in the PT group alone averaged 7.4 sessions, while those in the PT + LI group averaged 8.7 sessions. The LI group received a single injection of 0.5 % lidocaine without epinephrine. Those in the PT + LI group received the injection first and began PT 2-3 days later. There was no difference in the outcome variables for the interventions performed alone or in combination at 1-month or 3-months post-treatment. Given the number of interventions administered, it is challenging to identify those that might have been efficacious. One noteworthy component of this study was the permission to use pain medication as needed (36-53% of the participants used medication). This is a confounding variable which leave the results in question.
TrP Compression
In a critical overview of current concepts Dommerholt et al. [26] discouraged the use of the term “ischemic compression.” Since TrPs have been reported to be significantly hypoxic and possess a lower pH, attempts to induce more ischemia appear to be counterproductive. Hence the term “TrP compression” has been recommended. However, there was no attempt to explain the physiologic effort of the sustained force. Perhaps the compression does not produce an ischemic event but it does appear to result in a reactive hyperemia.
Reported to be a component of Shiatsu, TrP compression involves the application of light pressure initially and the slow increase over approximately 60 seconds. Although the application will aggravate symptoms, as pressure is maintained, a feeling of the muscle “giving way” or “melting” beneath your fingers occurs over the course of the next 60 seconds (Daniels, Ishmael, & Wesley, 2003). Fryer and Hodgson [27] examined the effects of manual pressure release on MTrPs in the upper trapezius (n=37). Random assignment into treatment and control groups included pre- and post- PPT measures with a dolorimeter. Treatment was comprised of 60 seconds of manual pressure at a level of 7/10 on a VAS. The results were a significant increase in PPT for the manual pressure treatment group vs. control (p<0.01).
The effect of compression on MTrPs of individuals who worked at least four hours per day on a computer was studied by Cagnie et al. [28]. After a four week control session, the participants (n=19) were treated with 1-minute of compression, twice per week for four weeks. Pre-, post-, and 6-month follow-up measures of pain, NDI, cervical ROM, and muscle strength revealed significant improvements after treatment. Although there was no change in NDI, the other three outcome measures (pain, mobility, strength) all improved.
Hanten et al. [29] assessed the effect of a home exercise program (HEP) of TrP compression followed by sustained stretch for treatment of MTrP. Subjects randomly assigned to one of two groups: five-day HEP of compression and stretching twice daily vs. control. Treatment encompassed compression performed with a TheraCane (Thera Cane, Denver, CO) until “release” (defined as letting go or melting) followed by static stretching of five muscles for 60-90 seconds each. Outcome measures were PPT, VAS, and the percentage of time in pain. There was a significant difference between the treatment and control groups for PPT and VAS but no difference between groups for the percentage of time in pain. Thus, compression and stretching were deemed effective for the treatment of MTrP. Likewise, the effect of compression using a Backnobber II device (Pressure Positive Company, Gilbertsvile, PA) on discomfort associated with MTrPs involved the identification of two MTrPs in the upper back of 28 participants [30]. One MTrP was treated with six repetitions of 30-sec TrP compression with the Backnobber II device. Treatments were rendered every other day for one week. The other MTrP served as the control. PPT was assessed with a JTECH algometer. The results are revealed in Table 2. There was a significant increase in PPT for the treatment group.
Table 2. Pain Pressure Threshold for Compression with Backnobber II [30]
|
Pre-Test
Mean ± SD (N) |
Post-Test
Mean ± SD (N) |
Treated MTrP |
31.73 ± 11.28 |
44.02 ± 13.31 |
Non-Treated MTrP |
34.10 ± 12.72 |
31.83 ± 11.65 |
Hou et al. [31] conducted a two-stage evaluation of the compression technique followed by a compilation of several modalities. Stage 1 implemented two magnitudes of pressure (pain threshold & average of pain threshold & tolerance) across three durations (30, 60, 90-seconds). The results of stage 1 were the pain reduction was statistically greater when using pressure of average pain threshold and tolerance pressures for 90-seconds. Stage 2 employed six combinations of treatments: 1) hot pack (HP) for 20-minutes with AROM of cervical spine; 2) HP, AROM and IC; 3) HP, AROM, compression, and electrical stimulation (ES) at 100-Hz for 20-minutes; 4) hot pack, AROM, and vapocoolant; 5) hot pack, AROM, vapocoolant, and ES; 6) HP, AROM, interferential stimulation (IF) at 4000/4100-Hz for 20-minutes, and myofascial release (MFR) of the upper trapezius. Stage 2 revealed improvement in pain threshold in all treatment groups but ROM was better in the groups with vapocoolant (group 4), vapocoolant with ES (group 5), and IF with MFR (group 6).
Ultrasound
Therapeutic ultrasound (US) has the potential to influence MTrPs via the mechanical effects and/or the thermal effects. There are several studies which have explored various US parameters and their effect on MTrPs. Aguilera et al. [32] examined the immediate effect of US and compression techniques for MTrPs in the trapezius (n=66). The participants were divided into three groups, 90 seconds of TrP compression, 2-minutes of 1-MHz pulsed ultrasound at 1-W/cm2, and 5-minutes of sham ultrasound. Outcome measures included: AROM of the cervical spine, basal electrical activity (BEA) of the trapezius, and PPT. The results were an immediate decline in BEA and MTrP sensitivity after both compression and US. The compression group also improved in cervical ROM.
Effect of high-power pain threshold US in the elderly with latent MTrP was studied by Kim et al. [33] with 41 participants. At baseline, 1, 2, 3, and 4 weeks of time, VAS, PPT, and cervical ROM were assessed. The 1-MHz continuous US treatments to the two groups were classified as conventional (1 W/cm2 x 5 min with a 20 cm2 transducer, 40 cm2 treatment area moving at 2.5 cm/sec) and high-power pain threshold (static transducer at intensity to threshold x 4-5 sec and then ½ the intensity x 15 sec; repeated 3 times). Treatments were rendered twice a week for four weeks (8 treatments). Both groups improved in VAS, ROM, and PPT but there was no significant difference between groups. Both of these studies used ultrasound parameters that could not achieve any appreciable heating effect yet they both resulted in improvement in the outcome measures (Table 3). Thus, the mechanic properties of acoustical streaming, increased phagocytosis, and degranulation of mast cells to produce a histamine release may have played a role [34].
Table 3. Conventional versus High-power, Pain-threshold Ultrasound Effect on Myofascial Trigger Points [33]
|
Baseline |
1 week |
2 week |
3 week |
4 week |
VAS |
US |
5.34 |
4.63 |
4.37 |
3.82 |
2.84 |
|
HP |
6.27 |
5.11 |
4.80 |
4.05 |
3.36 |
PPT |
US |
2.13 |
2.04 |
2.20 |
2.23 |
2.35 |
|
HP |
2.08 |
2.12 |
2.30 |
2.25 |
2.50 |
ROM |
US |
18.65 |
19.32 |
20.91 |
20.82 |
24.21 |
|
HP |
18.65 |
20.08 |
20.24 |
22.74 |
23.02 |
On the contrary, Draper et al. [35] examined the thermal US effects on MTrPs in the upper trapezius muscles. They examined 26 patients, randomly assigned to US or sham. The 3-MHz US treatment was performed at 1.4 W/cm2 for 5-minutes using a 7 cm2 US transducer over a 14 cm2 area. Two treatments were administered over 2 weeks and a dolorimeter was used to assess PPT. The data revealed a significant difference for both the immediate and residual PPT between US and sham treatments (Table 4). The parameters of this study would have resulted in a vigorous thermal response (~4°C). The increased tissue temperature, collagen extensibility, blood flow, enzymatic activity, and/or pain threshold may have contributed to the reduction in MTrP discomfort [34,36].
Table 4. Influence of Thermal Ultrasound versus Sham on Pain Pressure Threshold of Myofascial Trigger Point [35]
|
Immediate
Effect |
Resident
Effect |
US |
+ 2.65 |
+ 2.09 |
Sham |
+ 0.64 |
+ 0.93 |
To further differentiate the thermal versus mechanical influence, Ilter et al. [37] studied pulsed versus continuous US on MTrPs. Sixty participants were randomly assigned to one of three groups: continuous 3-MHz US (1 W/cm2 x 5-minutes), 50% pulsed 3-MHz US, and sham. All treatments included moist heat (10-minutes) and therapeutic exercises for 5-days per week for two weeks. Outcome measures were VAS, NDI, Beck Depression Scale, and Nottingham Health Profile (quality of life scale). All groups showed improvement in all parameters but the continuous US group greater pain relief at rest. Again, the thermal effects seemed to have a positive influence but in this study, the use of moist heat and exercise may have confounded the effect of the US treatments. Nonetheless, the use of multiple modalities/techniques is common in clinical practice.
Two additional studies have used phonophoresis to address MTrPs. Gulick et al. [38] explored the influence of US with methyl nicotinate (MN) on MTrPs. Thirty individuals, each with four MTrPs in the back musculature were treated with four different interventions: 1) continuous 3-MHz US with Aquasonic gel at 1.0 W/cm2, 2) continuous 3-MHz US with MN at 1.0 W/cm2, 3) 20% pulsed 3-MHz US with Aquasonic gel at 1.0 W/cm2, and 4) sham US. All treatments were 7-minutes in duration. Only the continuous US treatment with MN revealed a statistically significant difference between the pre-test and post-test PPT measurements (p = 0.025). It could be hypothesized the MN may have improved the transmission of the acoustic beam to heighten the effects produced by continuous US [39]. Whereas, Ay et al. [40] examined the effect of 1-MHz US with diclofenac to US without diclofenac, and sham. There were 20 subjects per group and the US parameters were 1.5 W/cm2 for 10-minutes. Both treatment groups had less pain and more cervical ROM after treatment but there was no difference between the US with and without the diclofenac. One might draw the conclusion the diclofenac did not enhance the US effects or the thermal effects of the US increased the blood flow to carry away the diclofenac.
Another study compared US to laser and compression [41]. US treatment was 5-minutes in duration at an intensity of 0.1 to 1.5 W/cm2 (frequency was not identified). The therapeutic laser probe was 1-cm in diameter with a 904 nm wave length and was delivered in a pulsed mode to a dose of 74 mJ/cm2 (treatment time of 30 seconds). The compression group was treated with manual digital pressure that gradually increased over 90 seconds to as much as 20 to 30 pounds of pressure. Five treatments were administered over five days. The dependent variables were VAS for pain, the soft tissue tenderness grading scale, and active cervical lateral flexion. Measures were taken at baseline and after the fifth treatment. All groups demonstrated improvement in all measures from pre-test to post-test. However, the laser group improvements were greater than that of the US and compression groups. The US results were attributed to thermal effects, while the laser results were related to analgesic effects, accelerated protein synthesis, and increased blood flow and compression to reactive hyperemia.
US combined with massage and exercise was studied by Gam et al. [42]. Fifty-eight people were randomized across three groups. Group 1 was treated with US, massage, and exercise. Group 2 was treated with sham US, massage, and exercise, while group 3 was the control. The 1-MHz US treatment was delivered at an intensity of 3 W/cm2 for 3-minutes, pulsed at 25%. The massage technique was up to 10-minutes of transverse friction and there were six exercises rendered for the neck and shoulders. Outcome measures were number, size, and tenderness of MTrPs, as well as VAS and use of analgesics. After six weeks of treatment (two times per week), there was a decrease in the number of MTrPs in both groups 1 and 2 but no difference in VAS or use of analgesics. Given the intensity and duration of the US treatment, it is not surprising there was no difference between groups 1 and 2. The treatment was unlikely to produce a thermal effect and the 25% pulsing for only 3-minutes would result in very limited mechanical effects. Thus, the transverse friction massage and exercises were most likely more influential.
Electrical stimulation
There are a large variety of combinations of electrical stimulation parameters that could be employed to influence MTrPs. Conventional transcutaneous electrical stimulation (high rate, sensory intensity), brief intense stimulation (high rate, noxious intensity), or low rate/opiate pain control (low rate, noxious intensity) are just a few of the possibilities. Hsueh et al. [43] examined three treatment parameters: sham, sensory nerve stimulation at 60 Hz, and motor stimulation at 10 Hz. All treatments were for 20-minutes. Each of the three groups (n=60) was further divided in two groups: severe pain and mild/moderate pain. Dependent variables VAS, PPT, and cervical ROM were assessed pre- and post-treatment. In the severe pain subdivisions, VAS and PPT displayed more improvement at 60 Hz but cervical ROM had more improvement at 10 Hz. In the mild/moderate pain subdivision, VAS improved at both frequencies, PPT improved more at 60 Hz, and cervical ROM improved more at 10 Hz. Although none of the parameters used in this study fit into the three pain management theories, statistically significant results were reported. Across both levels of pain, the higher rate (60 Hz) had a greater influence on pain while the lower rate (10 Hz) seemed to influence ROM.
Another mode of electrical stimulation is iontophoresis, the use of an electrical current to facilitate the movement of an ionic substance through the skin. Kaya et al. [44] utilized a direct current with and without lidocaine to treat MTrPs (n=58). Ten sessions were provided at a 3-mA intensity for 10-minutes over four weeks. At a low dosage of 30-mA-min (recommended dosage is 40-80 mA-min), it is not surprising to find ROM increase and PPT and VAS decrease but not to a statistically significant level. Disability score did increase significantly in both groups.
Laser
LASER is an acronym for Light, Amplification by, Stimulated, Emission of, Radiation. There are several different types of lasers ranging from low level light-emitting diodes (LEDs) to high powered, class IV lasers that stimulate cell function. There is limited literature on this topic as it relates to the treatment of MTrPs. Manca et al. [45] explored the influence of low level laser therapy (LLLT) and ultrasound on MTrPs. The researchers assigned 60 people to one of five groups: 1) US (3-MHz x 1.5 W/cm2 for 12 minutes), 2) placebo US, 3) LLLT (18J per session), 4) placebo LLLT, and 5) control. Treatment was five sessions per week for two weeks. Outcome measures were PPT, pain rating, and cervical spine lateral flexion. After both reassessments, two weeks of treatment and 12 weeks of follow-up, all interventions were better than control but active treatments were not better than placebo treatments. When evaluating the parameters of the two modalities, LLLT dose per session was very low, however, the US parameters should have produced vigorous heating. Perhaps it was not the US parameters but rather the US device. Prior research has demonstrated the intensity inconsistencies of various ultrasound devices [46].
A review by Uemoto et al. [47] compared the impact of laser and dry needling on MTrP. The authors stated laser therapy improves local microcirculation, enhances the supply of oxygen to cells with hypoxia, and helps remove the waste products of cell metabolism. According to Venancio et al. [48], wavelengths in the infrared spectrum of 780–904 nm are responsible for greater penetration for the deactivation of MTPs. However, Ilbuldu et al. [49] obtained satisfactory results via a lower electromagnetic spectrum (632.8 and 730 nm) and stated dosage is a key factor. Venancio et al. [48] suggested dosage range from 50 to 100 J/cm2, depending on the pain (chronic or acute), pigmentation of the skin (amount of melanin), amount of adipose tissue, and type of tissue (keratinized or mucous).
Dry needling
Dry needling can be defined as the penetration of a needle through the skin without the inclusion of any medication. Kietrys et al. [50] published a systematic review and meta-analysis on the effectiveness of dry needling on upper quarter myofascial pain. They reviewed 12 studies. Three of four studies showed a large effect in immediate pain relief. While two studies did not find dry needling superior to lidocaine injections or acupuncture in immediate pain relief. When examining the influence four weeks post-treatment, two studies showed a strong effect, three studies found lidocaine injections to be superior, one study found dry needling superior to standard rehab, and another found dry needling superior to acupuncture. Boyles et al. [51] also performed a systematic review of 304 articles, of which 19 were analyzed. The summary revealed a significant decrease in pain in 15 studies when compared to baseline and nine studies demonstrated a decrease in pain when compared to control or another treatment group. Five studies showed improvement in ROM when compared to control and five studies exhibited improved scores on a variety of outcome indexes: Neck Disability Index, Oswestry Disability Index, Foot Function Index, Nottingham Health Profile, Foot Health Status Questionnaire, and Short Form (SF-36) Healthy Survey. The PEDro score averaged 7.5 across 19 studies.
Taping
There are a plethora of tapes available for everything from ankle stabilization to securing bandages. However, there are only two studies using tape for MTrPs. Gulick et al. [52] use of KinesioTex tape (KT) was based on the company’s premise that the application of tape from proximal to distal is for muscle facilitation and from distal to proximal for inhibition. Thus, a “star-shaped” tape application was used over one of two MTrPs. The other MTrP was used as the control. The outcome measure was PPT and was performed at baseline, after 3-days of taping, and 4-days after tape removal. Thirty-one participants completed the study but there was no statistically significant improvement in PPT with the use of this specific KinesioTape technique. Since the publication of Gulick et al. [52], a study by Vered [53] revealed the direction of tape application (proximal to distal vs. distal to proximal) failed to influence muscle force development. Although this technique was not efficacious for MTrPs, it does not mean that other tapes or other taping techniques could not be beneficial. In fact, a study by Ozturk et al. [54] taped the upper trapezius (n=40) using the previously described “inhibition” technique. They taped the upper trapezius from distal to proximal for three days, one day rest, and re-applied for three more days. The control group had a horizontal piece of KT applied to the upper back. VAS, PPT, and upper trapezius muscle strength were measured at baseline, immediately after taping, and one-month later. All three measures were better from baseline to post-taping for the KT group. The VAS score was also better from post-test to one-month post-treatment for the KT group. However, the authors were unable to articulate a mechanism for this improvement.
Instrument Assisted Soft Tissue Techniques (IASTT)
IASTT use special instruments with beveled edges to assist the clinician in the evaluation and mobilization of soft tissue. IASTT have been purported to enhance proliferation of extracellular matrix fibroblasts, improve ion transport, and decrease cell matrix adhesions. IASTT has been suggested for a variety of pathology: lateral epicondylitis, carpal tunnel syndrome, trigger thumb, and plantarfascitis, to name a few. However, the use of IASTT on MTrPs has only been explored in one study [55]. A randomized, controlled study of 29 participants identified two MTrPs; one for treatment and one as the control. The six treatments (2 per week for 3 weeks), utilized sweeping, swiveling, and fanning techniques for a total of 5-minutes. The outcome measure used was the PPT and was assessed at baseline and after the six treatments. There was a significant difference between the pre-test and post-test of the treated MTrP, as well as between the treated and control trigger point (Figure 2). Thus, the 5-minute intervention using these three IASTT techniques can effectively increase the PPT of a MTrP in six treatments over a three-week period of time. The effectiveness of any one technique and the optimal treatment duration need to be explored.
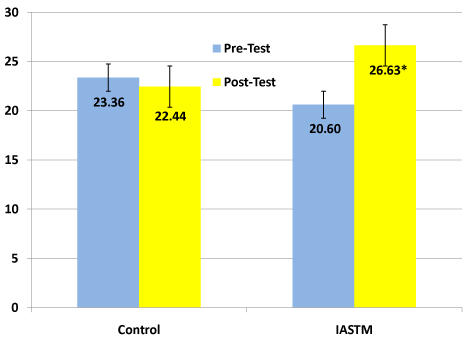
Figure 2. Comparison of pre-test and post-test pain pressure threshold values for the control and IASTM groups [55]
Conclusions
MTrPs have been associated with a decrescendo of events: excessive acetylcholine release at the motor endplates, compression of capillaries, decreased local blood flow leading to ischemic tissue, and limitation in oxygen and glucose availability resulting in an “energy crisis.” In addition to the identification and removal of perpetuating factors, the principal aim of this manuscript has been to identify the interventions used for the reduction of pain, recovery of normal muscle length, and increased range of motion for the deactivation of MTrPs. At this time, we do not know the “optimal” treatment for MTrPs. We have evidence for the use of lidocaine and diclofenac patches. We know TrP compression for 60-90 seconds is effective. We know that thermal US at vigorous heating levels (4° C), as well as phonophoresis with methyl nicotinate and diclofenac reduce pain. Electrical stimulation at 60 Hz and at 100 Hz reduces pain and at 10 Hz increases ROM. The majority of high-quality studies using trigger point dry needling showed a measurable benefit in multiple body areas. We also know IASTT may make a promising contribution. Whereas, treatment parameters for different types of laser and the various forms of taping are inconclusive. The potential for a summated effect should also be considered in the use of a multimodal approach. As demonstrated in some of the studies cited, pharmacologic therapies, various physical therapeutic modalities, and manual techniques in conjunction with one another may produce a more efficacious outcome.
Conflict of interest
This author reports there is no conflict of interest, monetary or otherwise, in the material presented in this manuscript.
References
- Majlesi J, Unalan H (2010) Effect of treatment on trigger points. Curr Pain Headache Rep 14: 353-360. [Crossref]
- Han SC, Harrison P (1997) Myofascial pain syndrome and trigger-point management. Reg Anesth 22: 89-101. [Crossref]
- Simons DG, Dommerholt J (2005) Myofascial pain syndromes – Trigger Points. Journal of Musculoskeletal Pain 13: 73-81.
- Alvarez DJ, Rockwell PG (2002) Trigger points: diagnosis and management. Am Fam Physician 65: 653-660. [Crossref]
- Myburgh C, Larsen AH, Hartvigsen J (2008) A systematic, critical review of manual palpation for indentifying myofascial trigger points: Evidence and clinical significance. Arch Phys Med Rehabil 89: 1169-1176. [Crossref]
- Shah JP, Phillips TM, Danoff JV, Gerber LH (2005) An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol (1985) 99: 1977-1984. [Crossref]
- Lavelle ED, Lavelle W, Smith HS (2007) Myofascial trigger points. Anesthesiol Clin 25: 841-851. [Crossref]
- Ingraham P (2016) Dance of the Sarcomeres. https://www.painscience.com/articles/dance-of-the-sarcomeres.php Jan 2014; Accessed May 26, 2016.
- Thomas K, Shankar H (2013) Targeting myofascial taut bands by ultrasound. Curr Pain Headache Rep 17: 349. [Crossref]
- Travell JG, Simon DG (1992) Myofascial pain & dysfunction: the trigger point manual. Volumes 1 & 2, Baltimore (MD): Williams & Wilkins
- Daniels JM, Ishmael T, Wesley RM (2003) Managing myofascial pain syndrome: sorting through the diagnosis and honing treatment. Phys Sportsmed 31: 39-45. [Crossref]
- Diakow PR (1992) Differentiation of active and latent trigger points by thermography. J Manipulative Physiol Ther 15: 439-441. [Crossref]
- Desai MJ, Bean MC, Heckman TW, Jayaseelan D, Moats N, et al. (2013) Treatment of myofascial pain. Pain Manag 3: 67-79. [Crossref]
- Vazquez-Delgado E, Cascos-Romero J, Gay-Escoda C (2010) Myofascial pain associated to trigger points: a literature review. Part 2: differential diagnosis and treatment. Med Oral Patol Oral Cir Bucal 15: 639-643. [Crossref]
- Vernon H, Schneider M (2009) Chiropractic management of myofascial trigger points & myofascial pain syndrome: a systematic review of the literature. J Manipulative Physiol Ther 32:14-24. [Crossref]
- Gebauer Company, http://www.gebauer.com/products/spray-and-stretch/spray-and-stretch-technique/. Accessed May 28, 2016.
2021 Copyright OAT. All rights reserv
- Ketenci A, Basat H, Esmaeilzadeh S (2009) The efficiency of topical thiocolchicoside (Muscoril) in the treatment of acute cervical myofascial pain syndrome: a single-blind, randomized, prospective, phase IV clinic study. Journal of the Turkish Society of Algology 21: 95-103.
- Rauck R, Busch M, Marriott T (2013) Effectiveness of a heated Lidocaine/Tetracaine topical patch for pain associated with myofascial trigger points: Results of an open-label pilot study. Pain Pract 13: 533-538. [Crossref]
- Affaitati G, Fabrizio A, Savini A, Lerza R, Tafuri E, et al. (2009) A randomized, controlled study comparing a lidocaine patch, a placebo patch, & anesthetic injection for treatment of TrP in patients with myofascial pain syndrome: evaluation of pain & somatic thresholds. Clinical Therapeutics 31: 705-720.
- Hsieh LF, Hong CZ, Chern SH, Chen CC (2010) Efficacy and side effects of diclofenac patch in treatment of patients with myofascial pain syndrome of the upper trapezius. J Pain Symptom Manage 39: 116-125. [Crossref]
- Avrahami D, Hammond A, Higgins C, Vernon H (2012) A randomized, placebo-controlled double-blinded comparative clinical study of 5 OTC topical analgesics for myofascial pain. Chiropr Man Therap 20: 7. [Crossref]
- Cho JH, Brodsky M, Kim EJ, Cho YJ, Kim KW, et al. (2012) Efficacy of a 0.1% capsaicin hydrogel patch for myofascial neck pain: a double-blinded randomized trial. Pain Med 13: 965-970. [Crossref]
- Borg-Stein J, Simons DG (2002) Focused review: myofascial pain. Arch Phys Med Rehabil 83: S40-47, S48-9. [Crossref]
- Baldry P (2002) Superficial versus deep dry needling. Acupunct Med 20: 78-81. [Crossref]
- Lugo LH, García HI, Rogers HL, Plata JA (2016) Treatment of myofascial pain syndrome with lidocaine injection and physical therapy, alone or in combination: a single blind, randomized, controlled clinical trial. BMC Musculoskelet Disord 17: 101. [Crossref]
- Dommerholt J, Finnegan M, Grieve R, Hooks T (2016) A critical overview of the current myofascial pain literature. J Bodyw Mov Ther 20:156-167. [Crossref]
- Fryer G, Hodgson L (2004) Effect of manual pressure release of myofascial trigger points in the upper trapezius muscle. Unpublished research from Victoria University, Melbourne Australia, December 15.
- Cagnie B, Dewitte V, Coppieters I, Van Oosterwijck J, Cools A, et al. (2013) Effect of TrP compression on trigger points in the neck & shoulder muscle in office workers: A cohort study. J Manipulative Physiol Ther 36: 482-489. [Crossref]
- Hanten WP, Olson SL, Butts NL, Nowicki AL (2000) Effectiveness of a home program of ischemic pressure followed by sustained stretch for treatment of myofascial trigger points. Phys Ther 80: 997-1003. [Crossref]
- Gulick DT, Palombaro K, Lattanzi JB (2011) Effect of Ischemic Pressure using a Backnobber II Device on Discomfort Associated with Myofascial Trigger Points. J Bodyw Mov Ther 15: 319–325. [Crossref]
- Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ (2002) Immediate effects of various physical therapeutic modalities on cervical myofascial pain & trigger-point sensitivity. Arch Phys Med Rehabil 83:1406-1414. [Crossref]
- Aguilera FJ, Martin DP, Masanet RA, Botilla AC, Soler LB, et al. (2009) Immediate effect of ultrasound and TrP compression techniques for the treatment of trapezius latent myofascial trigger points in healthy subjects: a randomized controlled study. J Manipulative Physiol Ther 32: 515-520. [Crossref]
- Kim Y, Yang HR, Lee JW, Toon BC (2014) Effects of the high-power pain threshold ultrasound technique in the elderly with latent myofascial trigger points: A double-blind randomized study. J Back Musculoskelet Rehabil 27:17–23. [Crossref]
- Prentice WE (2012) Therapeutic Modalities for Rehabilitation. (4thedn), McGraw-Hill, Columbus OH.
- Draper DO, Mahaffey C, Kaiser D, Eggett D, Jarmin J (2010) Thermal ultrasound decreases tissue stiffness of trigger points in upper trapezius muscles. Physiother Theory Pract 26: 167-172. [Crossref]
- Gulick DT (2008) Ultrasound (Book Chapter) Skeletal Muscle Damage & Repair. In: Peter M (Ed.), Tiidus, Human Kinetics, Champaign, IL February 2008.
- Ilter L, Dilek B, Batmaz I, Ulu MA, Sariyildiz MA, et al. (2015) Efficacy of pulsed & continuous therapeutic ultrasound in myofascial pain syndrome: a randomized controlled study. Am J Phys Med Rehabil 94:547-554. [Crossref]
- Gulick DT, Barsky J, Bersheim M, Katz K, Lescallette M (2001) Effect of Ultrasound on Pain Associated with Myofascial Trigger Points, Platform presentation APTA CSM, February 2001 (Abstract published in JOSPT 31(1), p A-19, January 2001).
- Gulick DT, Ingram N, Krammes T, Wilds C (2005) Comparison of Tissue Heating Using 3 MHz Ultrasound with T-Prep ® Versus Aquasonic ® Gel. Physical Therapy in Sport 6: 131-136.
- Ay S, Dogan SK, Evcik D, Baser OC (2011) Comparison of the efficacy of phonophoresis and ultrasound therapy in myofascial pain syndrome. Rheumatol Int 31: 1203-1208. [Crossref]
- Kannan P (2012) Management of myofascial pain of upper trapezius: a three group comparison study. Glob J Health Sci 4: 46-52. [Crossref]
- Gam AN, Warming S, Larsen LH, Jensen B, Hoydalsmo O, et al. (1998) Treatment of myofascial trigger-points with ultrasound combined with massage & exercise – a randomized controlled trial. Pain 77:73-79. [Crossref]
- Hsueh TC, Cheng PT, Kuan TS, Hong CZ (1997) The immediate effective of electrical nerve stimulation and electrical muscle stimulation on myofascial trigger points. Am J Phys Med Rehabil 76: 471-476. [Crossref]
- Kaya A, Kamanli A, Ardicoglu O, Ozgocmen S, Ozkurt-Zengin F, et al. (2009) Direct current therapy with/without lidocaine iontophoresis in myofascial pain syndrome. Bratisl Lek Listy 110: 185-191. [Crossref]
- Manca A, Limonta E, Pilurzi G, Ginatempo F, DeNatale ER, et al. (2014) Ultrasound and laser as stand-alone therapies for myofascial trigger points: A randomized, double-blind, placebo-controlled study. Physiother Res Int 19:166-175. [Crossref]
- Young R, Kimura IF, Gulick DT (1999) Accuracy of Intensity Output, Beam Nonuniformity Ratio, & Effective Radiating Area of Four Therapeutic Ultrasound Machines, Poster presentation NATA Annual Conference, June 1999 (abstract published in NATA Journal, April-June 34: S-69.
- Uemoto L, Garcia MA, Gouvêa CV, Vilella OV, Alfaya TA (2013) Laser therapy and needling in myofascial trigger point deactivation. J Oral Sci 55: 175-181. [Crossref]
- Venancio RA, Camparis CM/, Lizarelli RFZ (2002) Laser no tratamento de desordens temporomandibulares. JBA 2: 229–234.
- Ilbuldu E, Cakmak A, Disci R, Aydin R (2004) Comparison of laser, dry needling, and placebo laser treatments in myofascial pain syndrome. Photomed Laser Surg 22: 306-311. [Crossref]
- Kietrys DM, Palombaro KM, Azzaretto E, Hubler R, Schaller B, et al. (2013) Effectiveness of dry needling for upper-quarter myofascial pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther 43: 620-634. [Crossref]
- Boyles R, Fowler R, Ramsey D, Burrows E (2015) Effectiveness of trigger point dry needling for multiple body regions: a systematic review. J Man Manip Ther 23: 276-293. [Crossref]
- Gulick DT, Cain J, Cheney S, DeMarino D, Ettaro M, et al. (2015) Effects of Kinesio Tex Taping on Discomfort Associated with Myofascial Trigger Points. Orthopaedic Physical Therapy Practice.
- Vered E, Oved L, Zilberg D, Kalichman L (2016) Influence of kinesio tape application direction on peak force of biceps brachii muscle: A repeated measurement study. J Bodyw Mov Ther 20: 203-207. [Crossref]
- Öztürk G, Külcü DG, Mesci N, Şilte AD, Aydog E (2016) Efficacy of kinesio tape application on pain and muscle strength in patients with myofascial pain syndrome: a placebo-controlled trial. J Phys Ther Sci 28: 1074-1079. [Crossref]
- Gulick DT (2014) Influence of instrument assisted soft tissue treatment techniques on myofascial trigger points. J Bodyw Mov Ther 18: 602-607. [Crossref]


