Objective: Objective of the study is to examine whether Streptococcus mutans and Streptococcus sobrinus, which are main cariogenic bacteria existing in dental plaque attached to tooth surface at each site, exist site-specifically or not.
Method: From 9 male subjects, dental plaque accumulated for two days were collected from teeth surface at 8 sites. Detection rate, DNA concentration and component ratio of S. mutans and S. sobrinus in dental plaque collected from each tooth surface was examined using Real-time PCR method.
Results: With statistically significant difference observed in component ratio of S. mutans, LPB and LAL showed the highest and lowest value, respectively (p <0.05).
Conclusions: It has been suggested that bacteria flora of the mouse in dental plaque collected from each tooth surface has site specificity.
site specificity, cariogenic bacteria, dental plaque
Indexes for assessing cariogenic potential of dental plaque include pH (acid production ability of dental plaque), buffer capacity, bacterial species and numbers, and their composition ratio. Among these indexes, buffering ability is believed to affect solution environment around teeth surface including saliva and dental plaque fluid as well as to play an important role in determining dissolution velocity of tooth substance and remineralization reaction [1,2]. Main cariogenic bacteria is mutans streptococci among others [3,4], in particular, Streptococcus mutans and Streptococcus sobrinus are bacteria requiring greatest attention because they are isolated from human oral cavity and excellent in acid production ability and acid resistant ability as well [5-9].
From examinations of pH, buffer capacity and acid production abilities of dental plaque at each site attached to the labial and lingual (palate) surfaces of upper and lower anterior teeth region, buccal and lingual (palate) surfaces of posterior teeth region, i.e. 8 sites in total, we have clarified that dental plaque attached to lingual surface of lower anterior teeth region is rich in F ion [10-12] with high pH and buffer capacity [13,14] and low acid production ability [15]. These biochemical specificities in oral cavity site in cariogenesis are consistent with the fact that caries is epidemiologically found less in lower anterior teeth [16].
As a report on bacteriologic examination of dental plaque attached to each tooth surface, on the other hand, Ikeda, et al. [17] have reported that number of S. mutans in dental plaque attached to each tooth surface of occlusal fossa fissure was the largest among those attached to each tooth surface of buccal surface, approximal surface, and occlusal fossa fissure of mandibular first molar they examined. However, no study has examined on the labial and lingual surfaces of upper and lower anterior teeth region as well as buccal and lingual surfaces of molar region.
Colony count method has been widely adopted for quantitative assessment of bacteria up to now in which bacteria are cultured in a culture media and formed colonies are counted. The method requires several days to obtain the result while it is possible to count aerobic viable alone. However, real-time PCR method has spread as a new quantitative method in recent years because of its higher detection sensitivity [18] than colony count method and capability to obtain results promptly. In the dental field, real-time PCR method was conducted for the first time in 2000 by Lyons, et al. [19] for Porphyromonas gingivalis and in 2002 by Yano, et al. [20] and Fujiwara, et al. [21] for S. mutans as caries pathogen. Further, Yoshida, et al. [22] have reported they conducted quantitative method for S. sobrinus in 2003. However, there has been no report on examination of S. mutans and S. sobrinus in dental plaque attached to each tooth surface by using real-time PCR method.
Therefore, in order to clarify whether site specificity in the oral cavity in cariogenesis is reflected bacteriologically, level of S. mutans and S. sobrinus in dental plaque, which were attached to the labial and lingual surfaces of upper and lower anterior teeth region, buccal and lingual surfaces of posterior teeth region, i.e. 8 sites in total, was comparatively investigated for each site using real-time PCR method in the present study.
Experiment subjects
Experiment subjects were 9 male adults (aged from 23 to 26) volunteers. Upon obtaining consent from them in advance after explaining intent of the study, clinical examination of oral cavity and a questionnaire survey on oral prophylaxis time were conducted. DMFT of the experiment subjects was 7.9 ± 2.1 and all of them used dentifrice containing fluoride and lived in regions where fluoride had not been added to tap water until then.
The following items to be kept were sufficiently explained to volunteers prior to the experiment.
- To perform PMTC three days before dental plaque collection
- To suspend oral prophylaxis such as brushing from two days before dental plaque collection
- To record what they drank and ate during the period of oral prophylaxis suspension
- No to take anything except for water in the morning on the dental plaque collection day.
- The current study has obtained approval (No. 2014-4) from Ethical Committee of the University.
Dent a plaque collection method
Upon confirming by interviewing volunteers whether or not they have kept the instruction before dental plaque collection, dental plaque of day 2 was collected from upper anterior buccal (UAB), upper anterior lingual (UAL), upper posterior buccal (UPB), upper posterior lingual (UPL), lower anterior buccal (LAB), lower anterior lingual (LAL), lower posterior buccal (LPB) and lower posterior lingual (LPL) surfaces as soon as possible using a sterile sickle-shaped scaler (No.5/6) under simple exclusion of moisture while sucking saliva using a vacuum device on a dental unit. Dental plaque collected by the scaler was filled and sealed in a plastic micro tube with a capacity of 1.5 ml of which weight had been measured in advance, and then the wet weight was measured.
Extraction of DNA in dental plaque samples
Centrifugal washing was conducted for dental plaque samples collected at each site in the micro tube by adding normal saline and QIAamp® DNA Mini Kit(Qiagen) was used for DNA extraction. Extracted DNA was kept at -20℃ after determining the quantity by a spectrophotometer (260 nm).
Real-time PCR method
Primer and probe were targeted at glucocyltransferase-B in S. mutans and glucocyltransferase-T in S. sobrinus, respectively. In the current study, primer and probe of which bacterial species specificity had been confirmed by Yano, et al. [20] were used (Table 1).
Table 1. Primes and probes used in this study.
Designation |
Sequence |
Amplicon size(bp) |
Target |
Primers |
|
|
|
Smut3368‐F |
5'‐GCCTACAGCTCAGAGATGCTATTCT‐3' |
114 |
gtfB |
Smut3481‐R |
5'‐GCCATACACCACTCATGAATTGA‐3' |
|
|
Ssob287‐F |
5'‐TTCAAAGCCAAGACCAAGCTAGT‐3' |
88 |
gtfT |
Ssob374‐R |
5'‐CCAGCCTGAGATTCAGCTGT‐3' |
|
|
Uni152‐F |
5'‐CGCTAGTAATCGGATCAGAATG‐3' |
69 |
16S rRNA |
Uni220‐R |
5'‐TGTGACGGGCGGTGTGTA‐3' |
|
|
Fluorescent probes |
|
|
|
Smut3423T |
5'‐FAM‐TGGAAATGACGGTCGCCGTTATGAAA‐TAMRA‐3' |
|
gtfB |
Ssob298T |
5'‐FAM‐CCTGCTCCAGCGACAAAGGCAGC‐TAMRA‐3' |
|
gtfT |
Uni177T |
5'‐FAM‐CACGGTGAATACGTTCCCGGGC‐TAMRA‐3' |
|
16S rRNA |
The real-time PCR method was conducted using a template with TaqMan Universal PCR Master Mix (Applied Biosystems), 200 nM of primer and 250 nM of probe with Applied Biosystems 7500 by a condition to perform a cycle of operation for 2 minutes at 50℃, 10 minutes at 95 ℃, 15 seconds at 95 ℃ and a minute at 58 ℃ by 60 cycles as the condition [22].
Quantitative measurement of DNA concentration
Determination of DNA concentration of S. mutans and S. sobrinus was performed based on calibration curve method using real-time PCR method by preparing standards for the both bacteria. Measured DNA concentration was calculated based on unit dental plaque weight (ng/ml/mg). Detection limit by PCR method was 10 pg/ml for both S. mutans and S. sobrinus. In case of sites equal to or less than the detection limit, their concentrations were deemed to be 0.
Relative determination of cariogenic bacteria in dental plaque
Relative determination of S. mutans and S. sobrinus in dental plaque was conducted for the total bacteria quantity in the dental plaque by using Universal primer and Universal probe designed based on 16S rRNA gene conservation region which are common to all bacteria (Table 1). In reference to a formula suggested by Yoshida, et al. [22,23] and adopting it as an internality control, number of cycles (Ct) taken to reach threshold line, i.e. ⊿ Ct 16S rRNA was measured in the study. ⊿ Ct Target for S. mutans and S. sobrinus was measured respectively.
Component ratio of S. mutans and S. sobrinus in total number of bacteria in dental plaque was calculated using a formula; N = 1/2(⊿ Ct target - ⊿ Ct 16S rRNA) × 100 (%).
Statistical analysis
In order to check whether S. mutans and S. sobrinus in dental plaque is detected or not at each site for 9 subjects, Chi-squared test was conducted by dividing groups in which bacteria was detected and not detected into two groups by site. Comparison of DNA concentration between S. mutans and S. sobrinus at each site was examined using One-way ANOVA. Comparison of component ratio between S. mutans and S. sonrinus was examined by Friedman test and multiple comparisons were conducted for those that significance was suggested by Wilcoxon rank test.
Detection rate of cariogenic bacteria in dental plaque at each site
Figure 2 shows detection rate of S. mutans and S. sobrinus in dental plaque at each site. Even though significant difference was not observed, detection rate of UPB and LPB was the highest in 8 out of 9 subjects and that of LAL was the lowest in 5 out of 9 subjects in case of S. mutans. In case of S. sobrinus, resulting detection rate was lower at all sites compared with S. mutans.
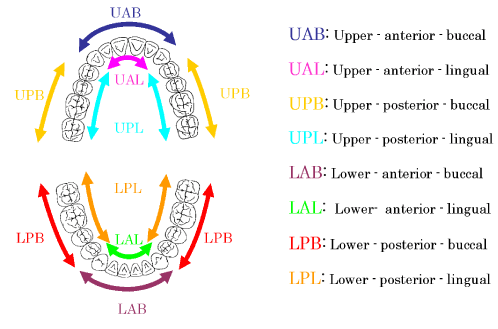
Figure 1. The sites of plaque collection
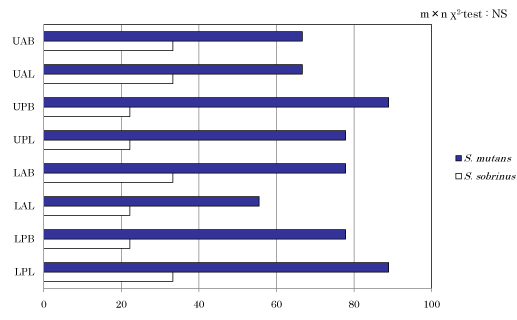
Figure 2. Detection of S. mutans, S. sobrinus in the sites.
DNA concentration of cariogenic bacteria in dental plaque at each site
Figure 3 shows result of DNA concentration measurement of S. mutans and S. sobrinus at each site. Even though significant difference was not observed in both of them, DNA concentration at LPB and LAL showed the highest and lowest concentration, respectively in case of S. mutans. In case of S. sobrinus, resulting DNA concentration at LPB and LAB was the highest and lowest, respectively.
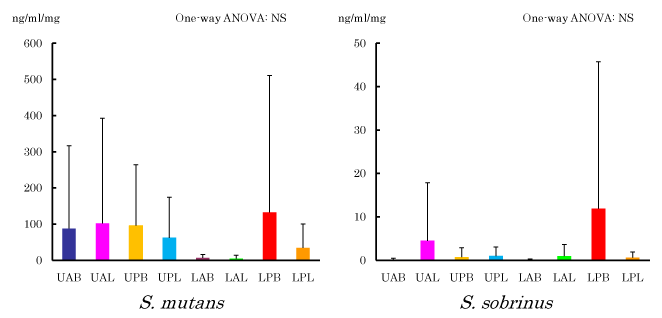
Figure 3. Absolute concentration of S. mutans and S. sobrinus
Component ratio of S. mutans and S. sobrinus in dental plaque at each site
Figure 4 shows component ratio of S. mutans and S. sobrinus in all bacteria in dental plaque. Statistical significance was observed in S. mutans with a hazard ratio of 5%. In Wilcoxon test, statistically significant difference was recognized between LAL which showed the lowest and other groups of UAB, UPB, LPB and LPL (p <0.05). In case of S. sobrinus, no sifnificnt difference was observed but the result was the highest in LPB and lowest in LAB, respectively.
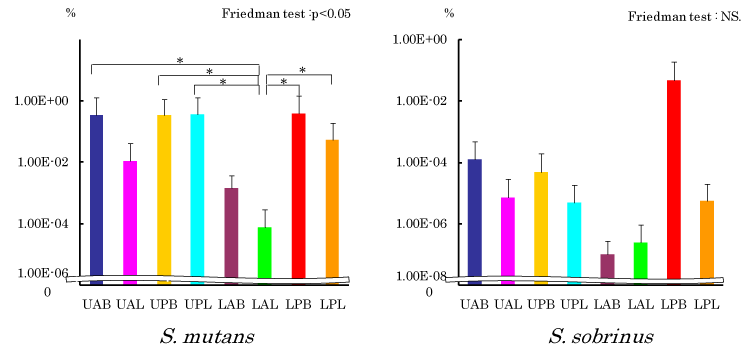
Figure 4. Relative concentration of S. mutans and S. sobrinus. Wilcoxon rank: *p<0.05
Detection rate of S. mutans and S. sobrinus in dental plaque at each site
Detection rate of S. mutans in dental plaque at each site was the highest in UPB and LPL (88.9%) and the lowest in LAL (55.6%). In case of S. sobrinus, on the other hand, detection rate was low at all sites. These results were consistent with those of Milgron, et al. [24] who reported that isolation frequency was lower in S. sobrinus than S. mutans and of Yoshida, et al. [22] who reported that detection rate was lower in S. sobrinus than S. mutans.
De Soet, et al. [9] who compared S. mutans and S. sobrinus in dental plaque have reported that continuous acid production ability is higher in S. sobrinus under starving environment. In addition, they have reported that S. sobrinus has abilities to form cariogenic dental plaque of compact structure with high protection capability and to produce lactic acid by adhering to smooth surface and colonizing thereon depending on sucrose, concluding that cariogenic potential of S. sobrinus is stronger. Further, in consideration of a report that subjects with S. mutans alone detected are more prone to caries than those with both S. mutans and S. sobrinus detected [25,26], it was believed that subjects with S. sobrinus detected have higher risk of caries.
DNA concentration and component ratio of cariogenic bacteria in dental plaque at each site
In the current study, as the results of examination of DNA concentration of cariogenic bacteria in dental plaque at each site, i.e. difference in absolute quantification, and component ratio of cariogenic bacteria in dental plaque at each site, i.e. relative quantification, no significant difference was recognized neither in S. mutans nor in S. sobrinus. The reason was assumed because samples used this time were dental plaque with a maturing period shortened by suspending oral prophylaxis such as brushing for two days. About 90% of bacteria in dental plaque colonized on the tooth surface become to be shared by Gram-positive bacteria and bacillus a day later and subsequently, thickness of the dental plaque grows by various bacteria to be attached thereon. From the fact that anaerobic bacteria increase along with maturation of dental plaque but that major part is dominated by chain coccus at any phase [27], it is assumed that significant difference was not observed by the site because maturing period was shorter for dental plaque of day 2 of the study.
With statistically significant difference observed in component ratio of S. mutans and S. sobrinus for total bacteria number in dental plaque, it was the highest in LPB and lowest in LAL, respectively. Causes to generate such site specificity are believed to include local existence of sucrose in the oral cavity. From a result of measurement of salivary clearance ability of glucose, Dawes, et al. have reported that LAL is a site with the highest and LPB is a site with the lower salivary clearance ability, respectively [28]. Therefore, it was assumed that LAL is a site difficult and LPB is a site, in contrast, easy to obtain sucrose required for producing glucan which is indispensable for S. mutans to become colonized and grow. In addition, in our examination in the past on pH of dental plaque at each site, LAL and LPB showed the highest and lowest value, respectively [13]. From this result, LPB was believed to be under an environment in which acid-resistant bacteria such as S. mutans are easy to become colonized.
While we have focused on S. mutans and S. sobrinus as cariogenic bacteria this time, about more than 500 types of bacteria are believed to exist in the oral cavity, and Streptococcus and Actinomyces mat become colonized on the teeth surface at early phase of dental plaque formation. As maturation of bacteria in dental plaque progresses, ratio of anaerobic bacteria increases [29,30]. In recent years, dental plaque has become to be called as biofilm because it clumps various bacteria to form a collective entity of them attached to the teeth surface surrounded by glycocalyx consisting mainly of polysaccharide produced on its own [30]. Therefore, in order to bacteriologically assess cariogenecity of dental plaque, it is important not only to examine existence and number of cariogenic bacteria in the dental plaque but also to recognize interaction between bacteria to cause caries and other bacteria within the biofilm. For example, Veillonella which elevates pH in dental plaque by consuming lactic acid, Streptococcus salivarius which produces ammonia as a pH elevation factor by hydrolyzing urea, and Actinomyces naeslundii [31] are believed to be in an antagonistic relationship with S. mutans and S. sobrinus [5-9] which are excellent in acid production ability and acid-resistant ability [31]. In addition, Streptococcus oralis, Streptococcus sanguinis and A. naeslundii which are involved in initial attachment to enamel [32,33] are usually observed on teeth surface without caries [33]. Mitzi, et al. have reported that significantly large number of S. sanguinis, S. mitis, S. oralis and Streptococcus pneumoniae were observed in dental plaque attached to healthy teeth and that significantly large number of S. mutans were observed in dental plaque attached to caries after examining bacteria in dental plaque attached to healthy teeth and to caries [34]. In other words, larger number of bacteria to attach to enamel initially exist in dental plaque with less cariogenic bacteria.
Therefore, it is necessary to focus not only on one of cariogenic bacteria, S. mutans alone but also on bacterial flora allowing for interfering relationship of bacteria to initially attach to and those to elevate pH in dental plaque.
Difference of bacterial flora in dental plaque by site has been examined by Handleman, et al. [35] and Ikeda, et al. [36] up to now. As a result of examination on difference of bacterial flora in dental plaque attached to lower anterior lingual surface, upper premolar approximal surface, and upper molar buccal surface, Handleman, et al. have reported that significantly larger number of Veillonella were observed on lower anterior buccal surface even though the number of bacteria itself was the same [35]. Ikeda, et al. have reported that difference in streptococci and Fusobacterium was significantly higher especially in occluding surface fissure compared with buccal surface as a result of examination of bacteria flora in dental plaque at lower first molar buccal surface, mesial surface and occluding surface fissure [36]. From these reports and results of the current study, as it is obvious that bacteria flora in dental plaque has site specificity, it is believed to be necessary to clarify not only cariogenic bacteria but also bacteria flora in dental plaque attached to each site in detail in the future.
It has been suggested that bacteria flora of the mouse in dental plaque collected from each tooth surface has site specificity.
This work was supported by JSPS KAKENHI Grant Number JP24792301.
- Tatevossian A (1990) Fluoride in dental plaque and its effects. J Dent Res 69: 645-652. [Crossref]
- Birkeland JM, Charlton G (1976) Effect of pH on the fluoride ion activity of plaque. Caries Res 10: 72-80. [Crossref]
- Fitzgerand RJ, Keyes PH (1960) Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc 61: 9-19. [Crossref]
- Orland FJ, Blayney JR, Harrison RW, Reyniers JA, Trexler PC, Wagner M, et al. (1954) Use of the germfree animal technic in the study of experimental dental caries. J Dent Res 33: 147-174. [Crossref]
- Takahashi N, Yamada T (1999) Acid-induced acid tolerance and acidogenicity of non-mutans streptococci. Oral Microbiol Immunol 14: 44- 48. [Crossref]
- Len ACL, Harty DWS, Jacques NA (2004) Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiol 150: 1339-1351. [Crossref]
- Takahashi N, Horiuchi M, Yamada T (1997) Effects of acidification on growth and glycolysis of Streptococcus sangius and Streptococcus mutans. Oral Microbiol Immunol 10: 72-76. [Crossref]
- De Soet JJ, Van Loveren C, Lammens AJ, Pavičić MJAMP, Homburg CHE, Ten Cate JM, et al. (1991) Differences in cariogenicity between fresh isolates of streptococcus sobrinus and streptococcus mutans. Caries Res 25: 116-122. [Crossref]
- Takahashi N (2002) Biochemical approach to dental plaque ecosystem. Tohoku Univ Dent J 21: 18-21.
- Hirose M, Matsumoto D, Yahata S, Igarashi S (2000) Site-specific of mineral ion in dental plaque. Jpn J Ped Dent 38: 965-971.
- Hirose M, Yahata S, Matsumoto D, Yahata S, Sakaguchi N, Tange T, et al. (2003) The relationship between site-specific mineral ion contents in dental plaque and salivary flow rates obtained from young adults. Ped Dent J 13: 17-21.
- Hirose M, Yahata S, Matsumoto D, Tange T, Igarashi S (2001) Site-specific relationship among Ca, P, F contents in dental plaque. Jpn J Ped Dent 39: 1010-1016.
- Hirose M, Atsushi F, Yahata S, Matsumoto D, Igarashi S (2005) Site-specificity of buffer capacity in dental plaque. J Dent Hlth 55: 15-21.
- Atsushi F, Hirose M, Yahata S, Matsumoto D, Igarashi S (2005) Site-specificity of pH and buffer capacity in dental plaque. Jpn J Ped Dent 43: 433-441.
- Yahata S, Hirose M, Fukuda A, Matsumoto D, Kutsumi S, Igarashi S (2007) Site-specificity of Acid Production in Dental Plaque. Jpn J Ped Dent 45: 531-535.
- Oral Health Association (2005) Comprehensive guide to the survey of dental diseases, edited the statistical analysis committee on the survey of dental diseases: 17-26.
- Ikeda T, Sandham HJ, Bradley Jr EL (1973) Changes in Streptococcus mutans and Lactobacilli plaque in relation to the initiation of dental caries in negro children. Archs oral boil 18: 555-566. [Crossref]
- Saukkoriipi A, Kaijalainen T, Kuisma L, Ojala A, Leinonen M (2003) Isolation of pneumococcal DNA from Nasopharyngeal samples for real-time, quantitative PCR. Mol Diagn 7: 9-15. [Crossref]
- Lyons SR, Griffen AL, Leys EJ (2000) Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J Clin Microbiol 38: 2362-2365. [Crossref]
- Yano A, Kaneko N, Ida H, Yamaguchi T, Hanada N (2002) Real-time PCR for quantification of streptococcus mutans. FEMS Microbiol Lett 217: 23-30. [Crossref]
- Fujiwara T, Hoshino T, Ooshima T, Hamada S (2002) Differential and quantitative analyses of mRNA expression of glucosyltransferases from Streptococcus mutans MT8148. J Dent Res 81: 109-113. [Crossref]
- Yoshida A, Suzuki N, Nakano Y, Kawada M, Oho T, Koga T (2003) Development of a 5’nuclease-based real-time PCR assay for quantitative detection of cariogenic dental pathogens streptococcus mutans and streptococcus sobrinus. J clin Microbiol 41: 4438-4441. [Crossref]
- Yoshida A, Suzuki N, Nakano Y, Oho T, Kawada M, Koga T (2003) Development of a 5’ fluorogenic nuclease-based real-time PCR assay for quantitative detection of Actinobacillus actionmycetemcomitans and Porphyromonas gingivalis. J clin Microbiol 41: 863-866. [Crossref]
- Milgron P, Riedy CA, Weinstein P, Tanner ACR, Manibusan L, Bruss J (2000) Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6-to 36-month-old children. Community Dent Oral Epidemiol 28: 295-306. [Crossref]
- Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, et al. (2005) Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J Med Microbiol 54: 661-665. [Crossref]
- Okada M, Soda Y, Hayashi F, Doi T, Suzuki J, Miura K, et al. (2002) PCR detection of Streptococcus mutans and S. sobrinus in dental plaque samples from Japanese pre-school children. J Med Microbiol 51: 443-447. [Crossref]
- Kolenbrander PE (2000) Oral Microbial Communities: Biofilms, interactions, and genetic systems. Annu Rev Microbiol 54: 413-437. [Crossref]
- Dawes C, Macpherson LMD (1993) The distribution of saliva and sucrose around the mouth during the use of chewing gum and the implications for the site-specificity of caries and calculus deposition. J Dent Res 72: 852-857. [Crossref]
- Ritz HL (1967) Microbial population shifts in developing human dental plaque. Archs Oral Biol 12: 1561-1568. [Crossref]
- Kolenbrander PE, London J (1993) Adhere Today, Here Tomorrow: oral bacterial adherence. J Bacteriol 175: 3247-3252. [Crossref]
- Burne R, Marquis RE (2000) Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett 193: 1-6. [Crossref]
- Paddick JS, Brailsford SR, Kidd EA, Gilbert SC, Clark DT, Alam S, et al. (2003) Effect of the environment on genotypic diversity of Actinomyces naeslundii and Streptococcus oralis in the oral biofilm. Appl Environ Microbiol 69: 6475- 6480. [Crossref]
- Ge Y, Caufuield PW, Fisch, GS, Li Y (2008) Streptococcus mutans and Streptpcoccus sanguinis colonization correlated with caries experience in children. Caries Res 42: 444-448. [Crossref]
- Mitzi R Becker, Bruce J Paster, Eugene J Leys, Melvin L Moeschberger, Sarah G Kenyon, Jamie L Galvin, et al. (2002) Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol 40: 1001-1009. [Crossref]
- Handelman SL, Hess C (1969) Bacterial populations of selected tooth surface sites. J Dent Res 48: 67-70. [Crossref]
- Ikeda T, Sandham HJ, Takai K (1980) A comparison of cultivable plaque microbiota on buccal surface, proximal area and occlusal fissures. Nihon Univ J Oral Sci 6: 341-347.




