Abstract
Background
Vitiligo is an acquired dermatologic disorder characterized by loss of functioning melanocytes. Several theories have been suggested that include; the genetic, autoimmune, neural, apoptotic, viral, self destruction and convergence theories. Protein gene product 9.5 (PGP 9.5) and Single strand DNA (ssDNA) are used as general neuronal and apoptotic markers respectively.
Objective
To study the possible contribution of either the neural mechanism or apoptotic mechanism or both together in the etiopathogenesis of generalized and segmental vitiligo variants.
Subjects and methods
This study included 20 vitiligo patients 10 segmental and 10 non segmental and twenty apparently healthy controls. Two biopsies were taken from each patient; one from the lesional and the other from the normally pigmented perilesional skin. One biopsy was taken from each control from non sun exposed skin. Biopsies were examined for the PGP 9.5 and ssDNA using immunohistochemical staining technique.
Results
A statistically significant difference between lesional segmental and non-segmental vitiligo compared to the control group (p<0.01) regarding PGP9.5 and ssDNA. A statistically significant difference was detected between lesional and non lesional skin as regards PGP9.5 (p<0.01) and ssDNA (p<0.01). A non statistically significant difference was detected on comparing staining intensity of the lesional segmental and non-segmental vitiligo as regards PGP9.5, yet, the results was statistically significant (P<0.01) as regard ssDNA .
Conclusion
Segmental and non-segmental vitiligo shares the neural theory, as the cutaneous axon terminals via chemical synapses make contact with epidermal melanocytes causing direct and indirect destructive effect on those cells. On the other hand, apoptosis is more evident in the non segmental type either through oxidative stress, autoimmune mechanism and/or any of the self destructive products of melanin synthesis, while in the segmental type the neural theory having the upper hand acting through apoptosis.
Key words
vitiligo, PGP9.5, ssDNA
Introduction
Vitiligo is an acquired dermatologic disorder characterized by loss of functioning melanocytes, resulting in depigmentation of the skin [1,2].
The mechanisms underlying the destruction of functioning melanocytes and the absence of melanin in vitiligo lesions remain unclear. Nevertheless, certain theories have been suggested and studied including; the genetic hypothesis, the autoimmune hypothesis, the neural hypothesis (involving neuropeptides, adrenergic and cholinergic neurotransmitters), the apoptotic theory, the viral hypothesis, the self destruction hypothesis (including the significant contribution of oxidative stress through the accumulation of H2O2), and convergence theory (which combines previous theories) [3-5].
The inheretence of vitiligo is polygenic with different loci or alleles. Most vitiligo susceptibility loci encode components of the immune system; and immune cells are found in the perilesional skin. Vitiligo often has autoimmune co- morbidity and it often respond to immunosuppressive treatments. Accordingly the autoimmune theory in vitiligo has the most advocates. Moreover, there is a speculation on the interplay between reactive oxygen species and the immune system in the pathogenesis of vitiligo [6-8].
Reactive oxygen species can induce the loss of dendricity of melanocytes, which not only could affect melanosome transfer but also could exaggerate traumatic transepidermal loss of genetically adhesion deficient melanocytes [9].
Cutaneous axon terminals and epidermal melanocytes make contact via chemical synapses in human skin. An increased release of chatecholamines from the autonomic nerve endings in the microenvironment of melanocytes in the vitiliginous areas has been reported. Norepinephrine is known for having direct and indirect melanocytotoxic effect. Direct actions include interacting with cellular sulfydryl groups, enzyme inhibition, impairing mitochondrial calcium uptake, and forming some cytotoxic productsincluding free radicals. The indirect effects include activating alpha receptors of the arteriols, causing a sever vasoconstriction, and thereby producing toxic oxygen radicals caused by hypoxia [10].
On the other hand accumulation of reactive oxygen species leads to melanocytes damage and the production of autoantigens through apoptosis [11].
Protein gene product 9.5 (PGP 9.5) is a general neuronal marker for all cutaneous sensory and autonomic nerve fibers. It has been studied in skin biopsies of various dermatologic disorders. PGP9.5 allows recognition of epidermal and dermal nerve fibers [12,13].
A monoclonal immunoglobulin M (IgM) antibody was used by Aroni et al., [14] against single strand DNA (ssDNA), which specifically stains the apoptotic cells and has been applied in vitiligo to differentiate between apoptotic and necrotic cells.
There is a relationship between the autonomic nerve system function and apoptosis, supporting the hypothesis that the destruction of functioning melanocytes in vitiligo could be the end result of different interacting pathogenic mechanism, such as apoptosis and accumulation of neural fibers/axons [14].
We aimed here to study the possible contribution of either the neural mechanism or apoptotic mechanism or both together in the etiopathogenesis of generalized and segmental vitiligo variants. This was done through immunohistochemical study of PGP9.5 as evidence of neural mechanism and ssDNA as an evidence of apoptotic mechanism in vitiligo.
Subjects and methods
This study included 20 vitiligo patients (11 males and 9 females) divided into groups according to the clinical type into segmental (10 patients; 4 males and 6 females) and non segmental (10 patients; 7 males and 3 females). Their age ranged from 21-35 years. Twenty age and sex matched healthy controls were included in the study. They were10 males and 10 females, and their age ranged from 24-45 years.
All patients and controls were subjected to full history taking, general examination and local examination of the patients skin lesions. A written informed consent was taken for participation in the study and approval of the local ethics committee was obtained.
Two biopsies were taken from each patient; one from the depigmented lesional area and the other from the normally pigmented skin at the periphery of the same area. One biopsy was taken from each subject of the control group from sun unexposed skin. Biopsies were collected and fixed in formalin 10% till time of the study.
Biopsies were examined for the PGP 9.5 and ssDNA using immunohistochemical staining technique.
Immunohistochmemical staining
Immunostaining for PGP9.5 and ssDNA were applied for all skin specimens. Immunohistochemical staining was done using Labeled StreptAvidin Biotin (LSAB) method in which the enzyme-labelled streptavidin reacts with the biotinylated secondary antibody. A kit supplied from Dako® LSAB+System-HRP, Carpinteria, California, USA was used for the substrates in this method. Purified mouse monoclonal antibody to PGP9.5 (BIOCARE MEDICAL, LLC., PGP9.5, CM 329 AK, Concord, California , USA) was used as the primary antibody to detect PGP9.5 in the tissue sections. Purified mouth monoclonal antibody to ssDNA (Enzo life sciences, International, INC, ssDNA, mAb (F7-26)R, ALX-804-192R, Plymouth Meeting, Pennsylvania, USA) was used as the primary antibody to detect ssDNA in the tissue sections. Incubation of the sections in Streptavidin-HRP solution (Dako®, Carpinteria, California, USA) was done for one hour at room temperature. This was followed by rinsing in PBS twice for 3 minutes each. Substrate-Chromogen solution (Buffererd substrate-DAB Chromogen, Dako®, Carpinteria, California, USA) was applied and incubated with the tissue sections for 10 minutes. This was followed by rinsing in distilled water twice for 3 minutes. Counterstainning was done by placing the slides in a bath of hematoxylin for 2 minutes followed by rinse in distilled water for 2 minutes. Dehydration of the sections was done through 95% ethanol for 1 minute then 100% ethanol for 2 minutes. This was followed by clearance in xylene for 2 minutes. The sections were mounted and coverslipped. The sections became ready for examination. A light microscope (Olympus CX5Japan) was used to examine the tissue sections under different magnification.
The immunostaining intensity was assessed semiquantitatively using an arbitrary scale, this scale was graded according to the intensity of staining from 1 to 3 as the following: 1=weak staining, 2=moderate staining, 3=intense staining. Three of the authors read the slides for intensity for every case with calculating the mean of their finding for every patient before applying the results for statistical analysis.
Statistical analysis
The collected data were revised, coded, tabulated and introduced to a PC using Statistical Package for Social Science (SPSS 15.0.1 for windows; SPSS Inc, Chicago, IL, 2001). Data were presented and suitable analysis was done according to the type of data obtained for each parameter. Mean Standard deviation (± SD) and range for parametric numerical data. Frequency and percentage of non-numerical data. Student T test was used to assess the statistical significance of the difference between two study group means. Chi-Square test: was used to examine the relationship between two qualitative variables. Fisher's exact test: was used to examine the relationship between two qualitative variables when the expected count is less than 5 in more than 20% of cells. Wilcoxon signed rank test: was used to assess the statistical significance of the difference of an ordinal variable (score) measured twice for the same study group. P- value: level of significance P>0.05: Non significant (NS), P< 0.05: Significant (S).
Results
Among the lesional biobsies there were 3 patients (15%) with +2 and 17 patients (85%) with +3 positivity staining for PGP9.5, while in the non lesional ones there were 11 patients (55%) with +1 and 9 patients (45%) with +2 positivity staining for PGP9.5. Lesional biopsies also showed that there were 12 patients (60%) with +2 and 8 patients (40%) with +3 positivity staining for ssDNA and the non lesional ones showed 18 patients (90%) with +1 and 2 patients (10%) +2 staining for ssDNA (Table 1 and Figures 1-6).
Table 1. Description of lesional and non lesional PGP9.5 and ssDNA among study cases
|
|
N
|
%
|
|
Lesional PGP9.5
|
+1
|
0
|
0%
|
|
+2
|
3
|
15.0%
|
|
+3
|
17
|
85.0%
|
|
Lesional ssDNA
|
+1
|
0
|
0%
|
|
+2
|
12
|
60.0%
|
|
+3
|
8
|
40.0%
|
|
Non lesional PGP9.5
|
+1
|
11
|
55.0%
|
|
+2
|
9
|
45.0%
|
|
+3
|
0
|
0%
|
|
Non lesional ssDNA
|
+1
|
18
|
90.0%
|
|
+2
|
2
|
10.0%
|
|
|
+3
|
0
|
0%
|
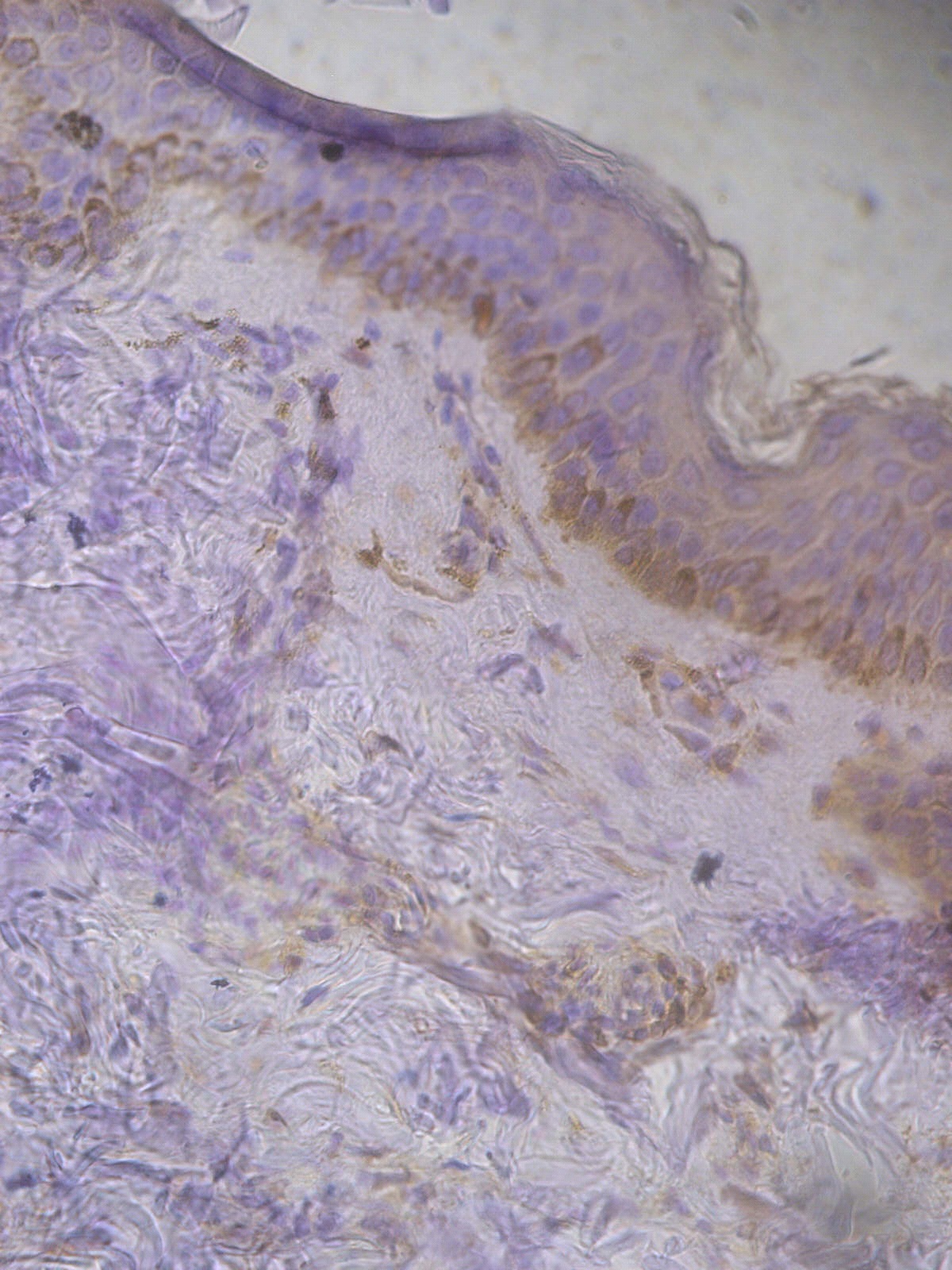
Figure 1. +1 dermal PGP9.5 positive staining in a non lesional segmental Vitiligo case (immunohistochemical stain, x400).
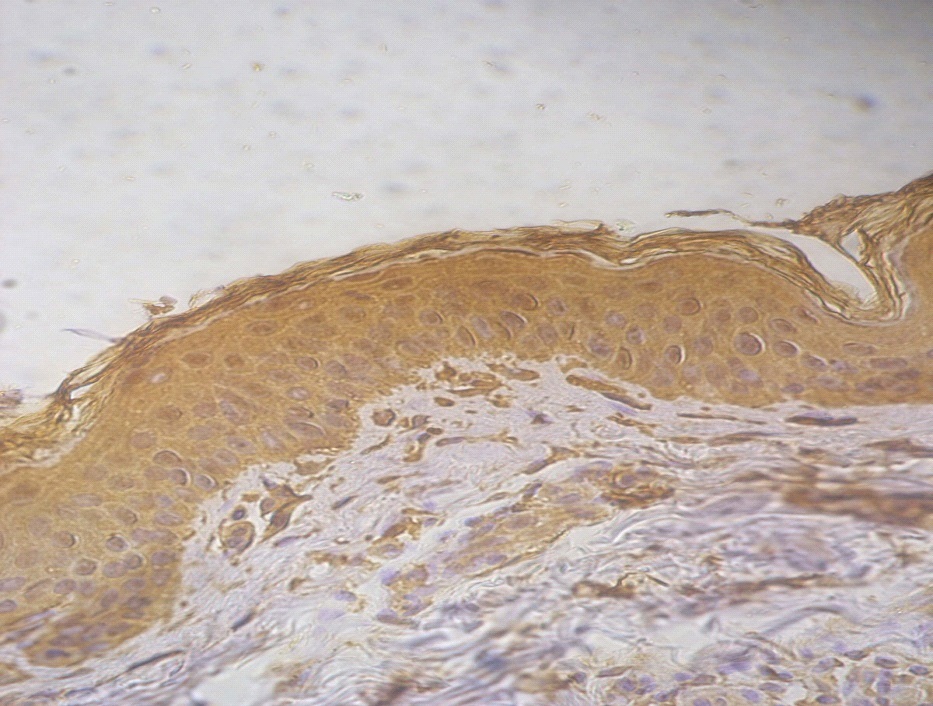
Figure 2. +2 dermal PGP9.5 positive staining in a lesional segmental Vitiligo case (immunohistochemical stain, x400).
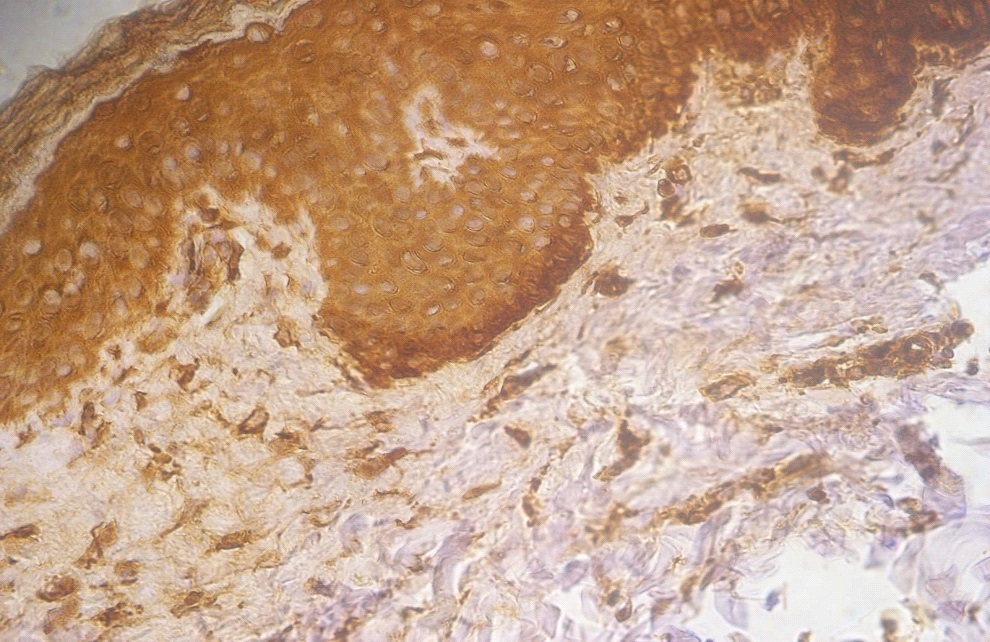
Figure 3. +3 dermal PGP9.5 positive staining in a lesional non segmental Vitiligo case (immunohistochemical stain, x400)
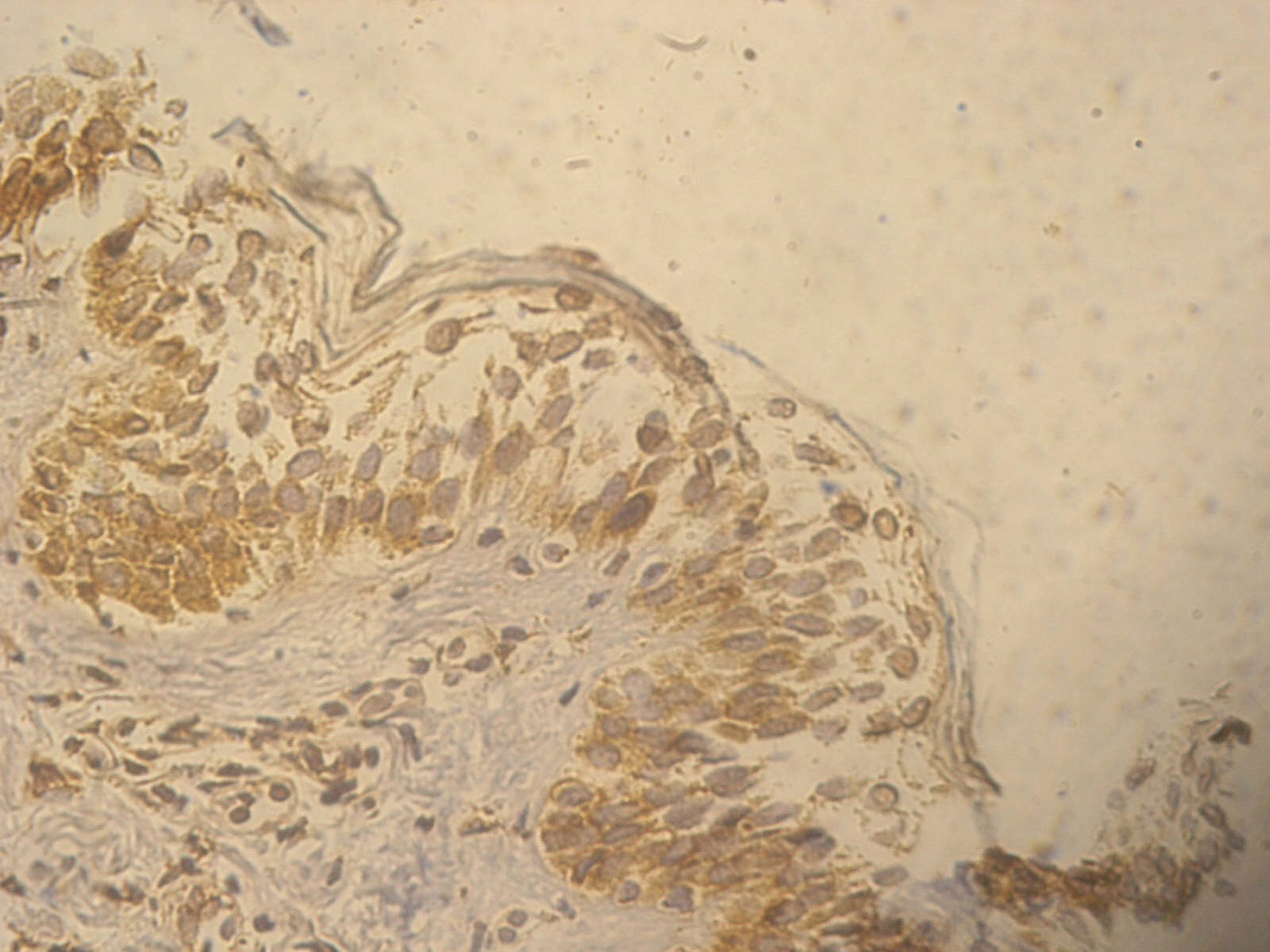
Figure 4. +1 ssDNA positive apoptotic epidermal cells in a non lesional segmental Vitiligo case (immunohistochemical stain, x400)
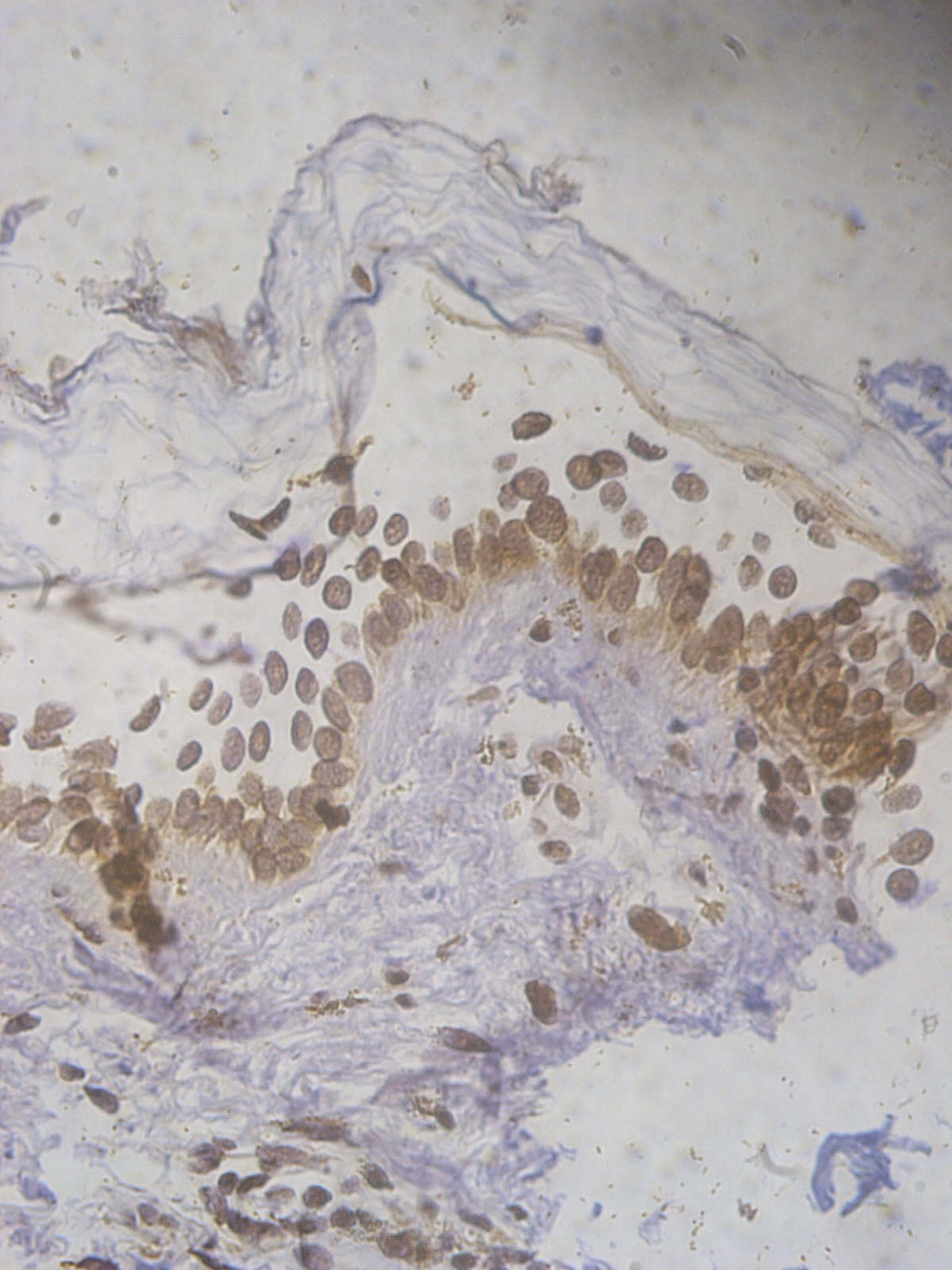
Figure 5. +2 ssDNA positive apoptotic epidermal cells in a lesional segmental Vitiligo case (immunohistochemical stain, x400).
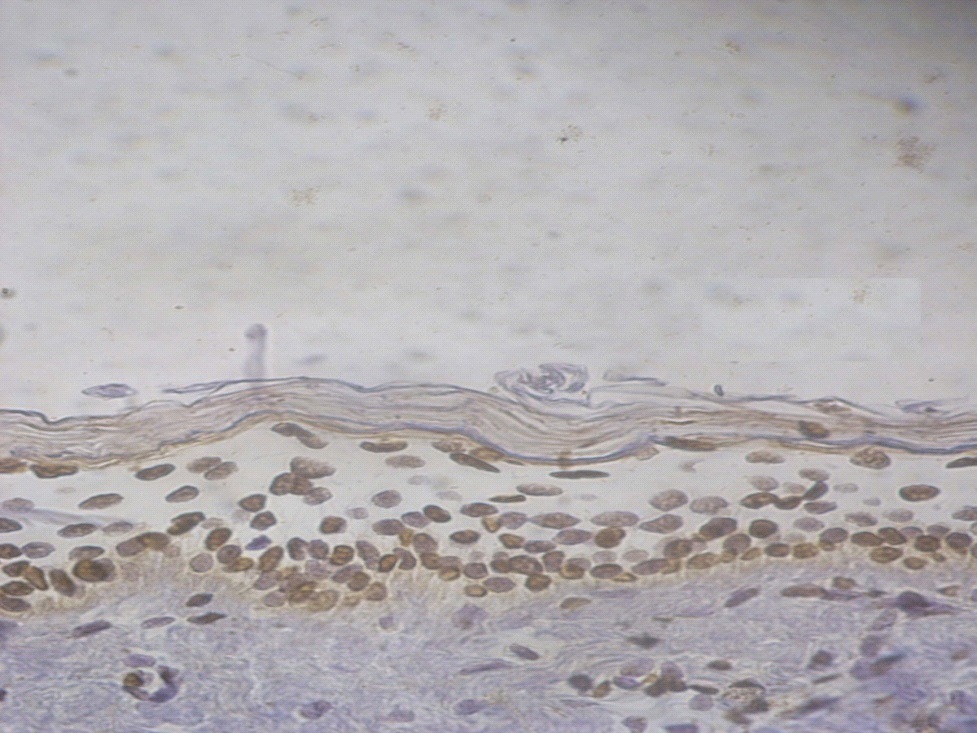
Figure 6. +3 ssDNA positive apoptotic epidermal cells in a lesional non segmental Vitiligo case (immunohistochemical stain, x400).
On comparing staining intensity of the lesional with non lesional skin of segmental vitiligo cases as regards PGP9.5, a high statistically significant difference was detected (P<0.01). Similar findings were detected comparing the lesional with nonlesional skin of non-segmental vitiligo cases with P<0.01 (Tables 2 and 3).
Table 2. Comparison between lesional and non lesional PGP9.5 among segmental vitiligo cases
|
Lesional segmental PGP9.5
|
Non lesional segmental PGP9.5
|
P
|
Sig
|
|
+1
|
+2
|
+3
|
+1
|
+2
|
+3
|
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
|
0
|
0%
|
3
|
30.0%
|
7
|
70.0%
|
6
|
60.0%
|
4
|
40.0%
|
0
|
.0%
|
0.004
|
HS
|
*Wilcoxon Signed Ranks Test
Table 3. Comparison between lesional and non lesional PGP9.5 among non-segmental vitiligo cases
|
Lesional Non segmental PGP9.5
|
Non lesional Non segmental PGP9.5
|
P
|
Sig
|
|
+1
|
+2
|
+3
|
+1
|
+2
|
+3
|
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
|
0
|
0%
|
0
|
.0%
|
10
|
100.0%
|
5
|
50.0%
|
5
|
50.0%
|
0
|
0%
|
0.004
|
HS
|
*Wilcoxon Signed Ranks Test
Among all vitiligo cases, a highly statistically significant difference was present between lesional and non lesional skin as regards positivity of PGP9.5 (85% of lesional skin were +3 positive compared to none of the non lesional skin) p <0.0 (Table 4).
Table 4. Comparison between lesional and non lesional PGP9.5 among all vitiligo cases
|
Lesional PGP9.5
|
Non lesional PGP9.5
|
P
|
Sig
|
|
+1
|
+2
|
+3
|
+1
|
+2
|
+3
|
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
|
0
|
0%
|
3
|
15.0%
|
17
|
85.0%
|
11
|
55.0%
|
9
|
45.0%
|
0
|
0%
|
0.0001
|
HS
|
*Wilcoxon Signed Ranks Test
There were also a highly statistically significant difference between lesional and non lesional skin as regards ssDNA (60% of lesional skin showed +2 staining compared to only 10% of non lesional) with (p <0.01) (Table 5).
Table 5. Comparison between lesion and non-lesion ssDNA among all vitiligo cases
|
Lesional ssDNA
|
Non lesional ssDNA
|
P
|
Sig
|
|
+1
|
+2
|
+3
|
+1
|
+2
|
+3
|
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
|
0
|
0%
|
12
|
60.0%
|
8
|
40.0%
|
18
|
90.0%
|
2
|
10.0%
|
0
|
0%
|
0.0001
|
HS
|
*Wilcoxon Signed Ranks Test
On comparing the lesional with non lesional segmental vitiligo cases as regards ssDNA, a high statistically significant difference was detected (P<0.01) with similar findings in the non-segmental vitiligo group (Tables 6 and 7).
Table 6. Comparison between lesional and non lesional ssDNA among segmental vitiligo cases
|
Lesional segmental l ssDNA
|
Non lesional segmental ssDNA
|
P
|
Sig
|
|
+1
|
+2
|
+3
|
+1
|
+2
|
+3
|
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
|
0
|
0%
|
10
|
100.0%
|
0
|
0%
|
10
|
100.0%
|
0
|
0%
|
0
|
0
|
0.002
|
HS
|
*Wilcoxon Signed Ranks Test
Table 7. Comparison between lesional and non lesional ssDNA among Non-segmental vitiligo cases
|
Lesional Non segmental ssDNA
|
Non lesional Non segmental ssDNA
|
P
|
Sig
|
|
+1
|
+2
|
+3
|
+1
|
+2
|
+3
|
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
N
|
%
|
|
0
|
0%
|
2
|
20.0%
|
8
|
80.0%
|
8
|
80.0%
|
2
|
20.0%
|
0
|
0%
|
0.004
|
HS
|
*Wilcoxon Signed Ranks Test
On comparing staining intensity of the lesional skin of segmental and non-segmental vitiligo cases as regards PGP9.5, a non-statistically significant difference was detected. While comparing the lesional skin of segmental and non-segmental vitiligo cases as regards ssDNA, a high statistically significant difference was detected P<0.01 (Tables 8 and 9).
Table 8. Comparison between Segmental and non-segmental vitiligo cases as regards lesional PGP9.5
|
|
Type
|
P*
|
Sig
|
|
Segmental Vitiligo
|
Non segmental Vitiligo
|
|
N
|
%
|
N
|
%
|
|
lesional PGP9.5
|
+1
|
0
|
.0%
|
0
|
.0%
|
.211**
|
NS
|
|
+2
|
3
|
30.0%
|
0
|
.0%
|
|
+3
|
7
|
70.0%
|
10
|
100.0%
|
Table 9. Comparison between Segmental and non-segmental vitiligo cases as regards lesional ssDNA
|
|
Type
|
P*
|
Sig
|
|
Segmental Vitiligo
|
Non segmental Vitiligo
|
|
N
|
%
|
N
|
%
|
|
lesional ssDNA
|
+1
|
0
|
.0%
|
0
|
.0%
|
.001**
|
HS
|
|
+2
|
10
|
100.0%
|
2
|
20.0%
|
|
+3
|
0
|
.0%
|
8
|
80.0%
|
On comparing staining intensity of the non lesional skin of segmental and non-segmental vitiligo cases as regards PGP9.5 and ssDNA, a non-statistically significant difference was detected (Table 10).
Table 10. Comparison between non lesional Segmental and non-segmental vitiligo cases as regards PGP9.5 and ssDNA:
|
|
Type
|
P*
|
Sig
|
|
Segmental Vitiligo
|
Non segmental Vitiligo
|
|
N
|
%
|
N
|
%
|
|
Non lesional PGP9.5
|
+1
|
6
|
60.0%
|
5
|
50.0%
|
1.00**
|
NS
|
|
+2
|
4
|
40.0%
|
5
|
50.0%
|
|
+3
|
0
|
.0%
|
0
|
.0%
|
|
Non lesional ssDNA
|
+1
|
10
|
100.0%
|
8
|
80.0%
|
.474**
|
NS
|
|
+2
|
0
|
.0%
|
2021 Copyright OAT. All rights reserv
2
|
20.0%
|
|
+3
|
0
|
.0%
|
0
|
.0%
|
Discussion
Several theories have been implicated in the etiopathogenesis of vitiligo [1,3-5]. In segmental vitiligo, neuronal mechanisms have suggested, as the central nervous system dampens excessive inflammatory responses by producing anti-inflammatory catecholamines and the inflammation is locally regulated by secretion of neuropeptides. As such, the neuronal hypothesis might be explained as a by-stander effect of inflammation instead of a triggering factor, where melanocytes become vulnerable to apoptosis. However, the pathophysiology of segmental vitiligo remains controversial [15].
Our study concerning segmental and non-segmental vitiligo and the role of neuronal and apoptotic theories in the pathogenesis of both, aiming to find out if segmental vitiligo is a single neuronal theory disease or there are other pathophysiological mechanisms leading to such a clinical presentation.
Our findings were consistent with the findings of Aroni et al., [14] that there was statistically significant difference between lesional and non lesional skin in the positivity of PGP9.5. Yet, different from that of Liu et al., [16] who reported that there was no statistically significant difference in PGP 9.5 positive nerve fibers between lesional and nonlesional skin in 18 patients with vitiligo. This could be attributed to differences in disease duration and disease activity, where our patients’ disease duration ranged from 1.3 to 7.6 years, in contrast to Liu et al., [16] whose study group having the duration of illness ranged from 2 month to 38 years. Moreover, more than 30% of his patients (6 patients out of the 18) had active vitiligo, developing new lesions 3 month prior to the study in contrast to our study where all the patients chosen with stable vitiligo. Maybe the more the stability of vitiligo, the less PGP 9.5 positive nerve fibers in the lesional skin and the more the disease duration the more the PGP9.5 postive nerve fibers in the lesional skin, which means by time the previously affected nerve may have the chance to partially regenerate and regain some of their lost functions.
To our current state of knowledge, no previous studies were performed on segmental vitiligo and PGP9.5 to support or oppose our findings. A statistically significant difference was detected between lesional and non lesional skin as regards PGP9.5 in all vitiligo cases. This suggests that autonomic/sensory nerve fibres directly participate in the pathogenesis of vitiligo more in the segmental group which is consistent with the belief that neuronal mechanisms play a role in the pathogenesis of segmental vitiligo.
A statistically significant difference (P<0.01) was found between lesional segmental and non-segmental vitiligo patients and the control group as regards PGP9.5, in contrary to the results obtained by Al’Abadie et al., [17] who showed no statistically significant difference in the papillary dermis in lesional and marginal skin of 12 vitiligo patients from control. Again this difference might be attributed to the difference in disease activity where 58% of patients in their study were active. These findings might reflect either a more destructive mechanism in the dermis at the lesion margin or an attempt to regenerate new fibers/axons in the dermis at lesional skin especially in segmental vitiligo cases.
We also detected no statistically significant difference on comparing the lesional skin of segmental and non-segmental vitiligo cases as regards PGP9.5. Both segmental and non-segmental vitiligo shares the neural theory, but apoptosis is more evident in the non segmental type as the difference between lesional skin of segmental and non-segmental vitiligo as regard ssDNA was statistically significant and more evident in the non segmental type.
Our results showed that, among all vitiligo cases, a statistically significant difference was present between lesional (segmental and non-segmental) and non lesional skin as regard ssDNA, yet the difference between non lesional segmental and non-segmental vitiligo patients and the control group was statistically non significant (p>0.05). We couldn’t compare our results to others as no previous studies compared the significance of staining intensity of ssDNA in the lesional and nonlesional skin. Our results here support the role of apoptosis in the pathogenesis of both types of vitiligo.
Among all our vitiligo cases there were complete absence of melanocytes in the lesions of segmental and non segmental cases which is consistent with the statistically significant difference between the 2 site (lesional and non lesional) in the staining of ssDNA in both groups. Accordingly, we could suggest that the apoptotic mechanism is dominantly operating in inducing vitiligo while additional mechanisms could augment the apoptotic mechanism in inducing vitiligo areas. Intense and moderate staining of ssDNA as an apoptotic marker in all cases highlights the importance of this apoptotic mechanism.
In our study statistically significant difference in the staining intensity of the lesional skin of segmental and non-segmental vitiligo cases as regards ssDNA , with higher staining intensity in non segmental cases, was detected. This may be due to the fact that the apoptotic theory plays higher role in non-segmental vitiligo than in the segmental type. On the other side, the neural mechanism through apoptosis in segmental vitiligo becomes more active to maintain the balance between apoptotic cells and still functioning cells. Meaning that any newly regenerated or transformed melanocytes from stem cells, become more susceptible to apoptotic destruction, explaining the resistant course of segmental vitiligo. However, to our current state of knowledge, no previous studies were performed comparing segmental vitiligo and non-segmental vitiligo as regards ssDNA to support or oppose our findings.
Finally, on the basis of the increased dermal PGP 9.5-positive nerve fibers and ssDNA-positive (apoptotic) cells we conclude and support that there is a possible relationship between autonomic nervous system function and apoptosis, supporting the hypothesis that the destruction of functioning melanocytes in vitiligo could be the end result of different interacting pathogeneic mechanisms, such as apoptosis and the accumulation of neural fibers/axon products, with the neural theory having the upper hand in segmental vitiligo through the induction of apoptosis. Accordingly, segmental vitiligo is not a single theory entity as the active neural component of the pathogenesis acts through the other theory (apoptosis).
Further research work on large scale of population is still needed in spite of the rarity of the segmental type to find out the exact sequence of events that leads to this type of the disease, presenting it in a different distribution and course than the non segmental type.
References
- Solano F, Briganti S, Picardo M, Ghanem G (2006) Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res 19: 550-571. [Crossref]
- van Geel N, Speeckaert M, Brochez L, Lambert J, Speeckaert R (2014) Clinical profile of generalized vitiligo patients with associated autoimmune/autoinflammatory diseases. J Eur Acad Dermatol Venereol 28: 741-746. [Crossref]
- Ortonne JP (2003) Vitiligo and Other Disorders of Hypopigmentation. In: Dermatology, Mosbey, London, Edinburgh, New York. 66: 947-952.
- Hasse S, Gibbons NC, Rokos H, Marles LK, Schallreuter KU (2004) Perturbed 6-tetrahydrobiopterin recycling via decreased dihydropteridine reductase in vitiligo: more evidence for H2O2 stress. J Invest Dermatol 122: 307-313. [Crossref]
- Schallreuter KU, Rübsam K, Gibbons NC, Maitland DJ, Chavan B, et al. (2008) Methionine sulfoxide reductases A and B are deactivated by hydrogen peroxide (H2O2) in the epidermis of patients with vitiligo. J Invest Dermatol 128: 808-815. [Crossref]
- Mohammed GF, Gomaa AHAl-Dhubaibi MS1 (2015) Highlights in pathogenesis of vitiligo. World J Clin Cases 3: 221-230. [Crossref]
- Iannella G, Greco A, Didona D, Didona B, Granata G, et al. (2015) Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. [Crossref]
- Laddha NC, Dwivedi M, Mansuri MS, Gani AR, Ansarullah M, et al. (2013) Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol 22: 245-250. [Crossref]
- Gauthier Y, Cario Andre M, Taïeb A (2003) A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 16: 322-332. [Crossref]
- Namazi MR (2003) Prescribing cyclic antidepressants for vitiligo patients: which agents are superior, which are not? Psychother Psychosom 72: 361-362. [Crossref]
- Xie H, Zhou F, Liu L, Zhu G, Li Q, et al. (2016) Vitiligo: How do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci 81: 3-9. [Crossref]
- Antunes SL, Liang Y and Neri JA (2003) The expression of NGFr and PGP 9.5 in leprosy reactional cutaneous lesions: an assessment of the nerve fiber status using immunostaining. Arq Neuropsiquiatr 61: 346–352. [Crossref]
- Ebenezer GJ, Daniel E (2004) Expression of protein gene product 9.5 in lepromatous eyes showing ciliary body nerve damage and a "dying back" phenomenon in the posterior ciliary nerves. Br J Ophthalmol 88: 178-181. [Crossref]
- Aroni K, Grapsa A, Lazaris AC, Kavantzas N, Kordosis T, et al. (2008) Tissue estimation of protein gene product 9.5 (PGP 9.5) expression and apoptosis in vitiligo. Int J Dermatol 47: 911-917. [Crossref]
- van Geel N, Mollet I, Brochez L, Dutré M, De Schepper S, et al. (2012) New insights in segmental vitiligo: case report and review of theories. Br J Dermatol 166: 240-246. [Crossref]
- Liu PY, Bondesson L, Löntz W, Johansson O (1996) The occurrence of cutaneous nerve endings and neuropeptides in vitiligo vulgaris: a case-control study. Arch Dermatol Res 288: 670-675. [Crossref]
- Al'Abadie MS, Senior HJ, Bleehen SS, Gawkrodger DJ (1994) Neuropeptide and neuronal marker studies in vitiligo. Br J Dermatol 131: 160-165. [Crossref]






