Abstract
Basal cell carcinoma (BCC), primarily affecting the photo-exposed areas of the body, is one of the most common skin cancers accounting for about 75% of all skin cancers. Various genetic and molecular factors, in addition to the exposure to ultraviolet (UV) B principally, play a role in the progression of BCC. Until now, the treatment of BCC relied on conventional cancer therapy measures such as surgical excision. However, enhanced understanding of the pathogenesis of the progression of BCC has increasingly intrigued the researchers to develop drugs targeting the molecular system. With the evolution in targeted therapy measures, immunotherapy is emerging as a potentially beneficial alternative. Activating or modulating the immune system to treat BCC is believed to demonstrate better and sustained outcomes. Three immunomodulatory drugs are currently approved by the FDA for the treatment of BCC - imiquimod, sonidegib and vismodegib, and several other are under investigations in clinical trials. The role of combination therapy (combining chemotherapy with immunotherapy) is also being explored. It is expected that immunotherapy may soon significantly transform the standard of care for BCC.
Key words
basal cell carcinoma, skin cancer, immunotherapy, immunomodulators, imiquimod, sonidegib and vismodegib
Abbreviations
AK: Actinic keratoses; ATM: Ataxia telangiectasia mutated; ATP: Adenosine triphosphate; BCC: Basal cell carcinoma; Bcl-2: B-cell lymphoma 2; CYLD: Cylindromatosis (turban tumor syndrome); CYP: Cytochrome P; DNA: Deoxyribonucleic acid; EDA: Ectodysplasin A; EDAR: Ectodysplasin-A receptor; EDAR: EDA receptor; EDARADD: EDAR-Associated Death Domain; FDA: Food and Drug Administration; Gli: Glioma-associated oncogene; GliA: Glioma-associated oncogene activated; GliR: Glioma-associated oncogene repressor; Hh: Hedgehog; I3A: Ingenol mebutate; IKK: IkB kinase; IRM: Immune response modifier; MDM2: Mouse double minute 2 homolog; NF-κB: Nuclear factor-κB; PD: Pharmacodynamics; PIK3: Phosphoinositide 3-kinase inhibitor; PK: Pharmacokinetics; PKC: Protein Kinase C; PTCH: Protein patched homolog 1; PUVA: Ultraviolet A with Psoralen; sBCC: Superficial basal cell carcinoma; SMO: Smoothened receptor; SMOH gene: Smoothened gene; t1/2: Half-life; TAB2: TAK1-binding protein 2; TAK1: Transforming growth factor beta-activated kinase 1; TRAF6: Tumor necrosis factor receptor associated factor 6; US: United States of America; UV: Ultraviolet
Introduction/epidemiology
Basal cell carcinoma (BCC) is a common type of non-melanoma skin carcinoma. According to the National Cancer Institute in 2014, more than 3.2 million new cases of non-melanoma skin cancers were diagnosed annually. Additionally, in the same year, less than 2000 cases of deaths were reported [1]. It is estimated that about 2.8 million BCC cases are diagnosed every year in the United States of America (USA) [2]. Australia has the highest incidence, followed by the USA and the least in Europe. Caucasians, Hispanics, and people native of Japan and China are more susceptible to develop BCC [3].
This cancer is caused primarily due to sun exposure. It is the most common type of skin cancer and accounts for 75% of all the skin cancers. In younger patients, its incidence is increasing continuously. In white populations, the incidence of BCC has doubled every 14 years, worldwide [4]. The age standardized incidence for BCC in whites residing in Southern USA is 300 per 100,000 per year. Around 3,000 deaths are reported every year in the USA due to advanced BCC [5]. It includes different types of histopathologic and genetic characteristics. There is a predominance of males over females by 30-80%, with higher incidence observed after the age of 40 years (as 96 % cases in the Australia) [4].
Etiology/Predisposing factors
There are many factors that increase the risk of BCC. The risk factors are as follows: [6]
- Exposure to intense sunlight: People who are exposed to direct sun for long time would be at increased risk. Younger people and people, who have at least one blistering sun burn, are reported to be at greater risk.
- Radiation exposure: Radiation like ultraviolet A with Psoralen (PUVA) treatments for psoriasis may be the risk factor for basal cell carcinoma.
- Fair skin: Fair skinned people are more on the verge of developing BCC than blacks.
- Sex: Men possess more risk than same aged women.
- Age: It generally develops in people older than 50 years. But children exposed more to ultraviolet (UV) radiation may be at higher risk.
- Family history: Past medical history of basal cell carcinoma in any age or positive family history makes an individual more susceptible to BCC.
- Effect of immunosuppressive treatments: Immunosuppressive medications, which are generally taken after organ transplantation, may increase the risk of skin cancer.
- Exposure to arsenic: People exposed to higher level of arsenic are at higher risk, like farmers and refinery workers.
- Some inherited syndromes: Some of the hereditary syndromes may also enhance the risk of BCC, like Nevoid basal cell carcinoma syndrome (Gorlin-Goltz syndrome) that causes pitting on the hands and feet and spine abnormalities, Xeroderma pigmentosum (loss of the ability to repair skin damage), causes an extreme sensitivity to sunlight.
Pathophysiology/Molecular basis
The progression of BCC is supported by various genetic factors, phototype of the skin specific to a person and exposure to UVB in a cumulative manner. Mostly, BCC arises as a sporadic tumor and, in very rare cases, as a hereditary syndrome. Taking the molecular basis into the consideration, BCC is represented by the atypical hedgehog signaling activation. This is due to the alterations in the protein patched homolog 1 (PTCH) or Smoothened (SMOH) genes. In around 50% of the cases, mutations occur in p53, a gene responsible for tumor suppression. These mutations result from C to T transitions mediated by UVB exposure [7]. Various signaling pathways and genes associated with BCC have been described below:
Hedgehog signaling pathway
The hedgehog (Hh) signaling pathway occurs during embryonic development. The name of this pathway is derived from the spiky processes of drosophila embryo. This pathway is started by the Hh ligand, which binds to the PTCH1 (present at the base of cilium, located on the cell membrane). This makes the Smoothened receptor (SMO) (present on endosomes) free, which further transmit signals. Then, SMO migrates from the intracellular endosome to the cell membrane of the cilium. This results in advancing the signal through various interacting proteins, leading to the activation of the Gli family of zinc finger transcription factors (glioma-associated oncogene Gli1, Gli2, and Gli3). This is followed by the conversion of repressor (GliR) to activated (GliA). GliA moves into the nucleus and leads to transcription of the target genes. However, when Hh is absent, SMO cannot generate further signalling, suggesting that Hh is important to start the hedgehog signalling and for cancer development [8]. It has been observed that in many cases of sporadic BCCs, SMO, which is responsible for the upregulation of the transcription associated with the target genes of hedgehog, is mutated [9]. In all those cases that lack PTCH mutations, a generalised mutation (Trp535Leu) occurs in the SMO transmembrane domain at the seventh position [10]. In brief, the PTCH activation or mutation of SMO is seen in almost all the cases of BCC. This advocates that dysregulation of hedgehog pathway is necessary for the formation of BCC [11].
NF-κB signalling pathway
Nuclear factor-κB (NF-κB) signaling pathway may be important in BCC. In this pathway, Ectodysplasin-A binds to its receptor (EDAR). A complex of EDARADD, tumor necrosis factor receptor associated factor 6 (TRAF6), Transforming growth factor beta-activated kinase 1 (TAK1)-binding protein 2 (TAB2) and TAK1 is then formed. After this, TAK1 activates IkB kinase (IKK). Activated IKK complex can cause ubiquitination and proteasome degradation of the inhibitory proteins IkB. NF-κB transcription factor may subsequently be released. This results in the translocation of NF-κB. The nucleus then activates the transcription of target genes. However, this pathway is terminated at TRAF 6 by the cylindromatosis (turban tumor syndrome) (CYLD); possibly by deubiquitinating. Thus, mutations in ectodysplasin A (EDA), EDA receptor (EDAR), EDAR-Associated Death Domain (EDARADD) and TRAF6 can all cause ectodermal dysplasia in humans. This may be the cause of the development of BCC (Figure 1) [12].
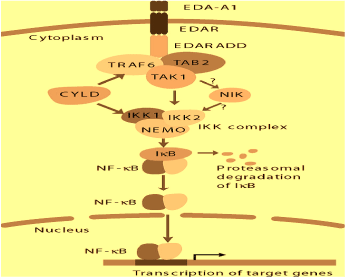
Figure 1. EDAR – NF-Kb Signalling pathway [6]
p53
The p53 gene is involved in the cell-cycle control and the maintenance of chromosomal stability. When deoxyribonucleic acid (DNA) damage occurs, p53 is phosphorylated by DNA damage-sensing proteins, such as Ataxia telangiectasia mutated (ATM). After this, the detachment of p53 from mouse double minute 2 homolog (MDM2) occurs along with stabilization and activation of target genes, regulated by p53. It is suggested that p53 may be involved in BCC development.
Although in normal skin wild type p53 is not present, it can be seen within 2 hours after UV irradiation, with peak levels at 24 hours after irradiation and again undetectable levels at 36 hours after irradiation. Moreover, p53 mutations have been detected in about half of all the BCCs [13].
p63
The role of p63 in BCC can be shown by an example, where abnormal expression of p63 can affect UVB-induced apoptotic pathway. Some isoforms have the capability to bind p53 consensus sequences and activate target genes. It is suggested that p63 may be involved in the progression of BCC [13].
Immunotherapy
Current immunotherapy options for basal cell cancer are discussed in following categories: Immunomodulators, SMO inhibitors, phosphoinositide 3-kinase (PIK3) inhibitors and other miscellaneous drugs.
Immunomodulators
Food and drug administration (fda) approved immunomodulators
Imiquimod: A synthetic agent with immune response modifying activity [14]. As an immune response modifier (IRM), imiquimod stimulates cytokine production, especially interferon production, and exhibits antitumor activity, particularly against cutaneous cancers. Imiquimod's proapoptotic activity appears to be related to B-cell lymphoma 2 (Bcl-2) overexpression in susceptible tumor cells.
Indications and use: Imiquimod is indicated for the topical treatment of non-hyperkeratotic, non-hypertrophic actinic keratoses (AK), Biopsy-confirmed, primary superficial basal cell carcinoma (sBCC) in immunocompetent adults, external genital and perianal warts/condyloma acuminata in patients of 12 years or older.
Pharmacodynamics/pharmacokinetics (PD/PK): The apparent half-life (t1/2) was seen 10 times more with topical application compared to the subcutaneous route (t1/2 -2 hours). It may be due to the long retention time during topical application. Mean peak drug concentration was found to be approximately 0.4 ng/ml.
Contraindications: It is contraindicated in patients with hypersensitivity.
Warnings: Skin weeping and erosion, exposure to sunlight, urinary swelling, malaise, fever, nausea, myalgias and rigors. If such types of toxicities occur discontinue the treatment.
Adverse events: Most common adverse reactions (incidence >28%) are application site reactions or local skin reactions: itching, burning, erythema, flaking/scaling/dryness, scabbing/crusting, edema, induration, excoriation, erosion, ulceration.
SMO Inhibitors
FDA approved SMO inhibitors
Vismodegib: A small molecule with potential antineoplastic activity. Vismodegib targets the Hedgehog signaling pathway, blocking the activities of the Hedgehog-ligand cell surface receptors PTCH and/or SMO and suppressing Hedgehog signalling [15]. The Hedgehog signaling pathway plays an important role in tissue growth and repair; aberrant constitutive activation of Hedgehog pathway signaling and uncontrolled cellular proliferation may be associated with mutations in the Hedgehog-ligand cell surface receptors PTCH and SMO.
Indications and use: Vismodegib is indicated for the treatment of metastatic basal cell carcinoma, or locally advanced basal cell carcinoma that has recurred following surgery. It is also used in those subjects that cannot go through the procedure of surgery or radiation.
PD/PK: The absolute bioavailability of vismodegib was found to be 31.8%. The volume of distribution of vismodegib was found to be 16.4 to 26.6 L. Its metabolism is done mainly through oxidation, glucuronidation, and pyridine ring cleavage. Its elimination half-life (t1/2) is found to be 4 days after continuous once-daily dosing.
Contraindications: It is contraindicated in patients with hypersensitivity.
Warnings: Teratogenic effects including midline defects, missing digits, and other irreversible malformations. So pregnancy status should be verified before starting of vismodegib. It might be exposed through semen, so females should use contraceptives.
Adverse events: The most common adverse reactions are muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea, decreased appetite, constipation, arthralgias, vomiting, and ageusia.
Sonidegib: A small molecule with potential antineoplastic activity. It targets the Hedgehog signaling pathway, blocking the activities of the Hedgehog-ligand cell surface receptors PTCH and/or SMO and suppressing Hedgehog signalling [16]. Sonidegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction.
Indications and use: It is indicated for the treatment of adult patients with locally advanced basal cell carcinoma (BCC) that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy.
PD/PK: The elimination half-life (t1/2) of sonidegib estimated from population pharmacokinetic (PK) modeling was approximately 28 days. Sonidegib is primarily metabolized by cytochrome P (CYP)3A. Sonidegib and its metabolites are eliminated primarily by the hepatic route.
Contraindications: It is contraindicated in patients with hypersensitivity.
Warnings: It can cause embryo-fetal death or severe birth defects when administered to a pregnant woman and is embryotoxic, fetotoxic, and teratogenic in animals. It might be exposed through semen, so females should use contraceptives.
Adverse events: The most common adverse reactions are muscle spasms, alopecia, dysgeusia, fatigue, nausea, musculoskeletal pain, diarrhea, decreased weight, decreased appetite, myalgia, abdominal pain, headache, pain, vomiting, and pruritus.
Non-FDA approved SMO inhibitors: The non-FDA approved SMO inhibitors have been mentioned in Table-1 below:
Table 1. Non-FDA Approved SMO Inhibitors [17]
Drug |
Clinical trial identifier no. |
Phase |
Study design |
Target |
Erismodegib |
NCT02303041 |
-- |
Efficacy Study/Open Label |
SMO |
Erismodegib: A small-molecule Smoothened (SMO) antagonist with potential antineoplastic activity. Erismodegib selectively binds to the Hedgehog (Hh)-ligand cell surface receptor SMO, which may result in the suppression of the Hh signaling pathway and hence, the inhibition of tumor cells in which this pathway is abnormally activated. The Hh signaling pathway plays an important role in cellular growth, differentiation and repair. Inappropriate activation of Hh pathway signaling and uncontrolled cellular proliferation, as is observed in a variety of cancers, may be associated with mutations in the Hh-ligand cell surface receptor SMO.
PIK3 Inhibitors
Non-FDA approved PIK3 inhibitors: The non-FDA approved PIK3 inhibitors have been mentioned in Table-2 below:
Table 2. Non-FDA Approved PIK3 Inhibitors[17]
PIK3 inhibitors |
Clinical trial identifier no. |
Phase |
Study design |
Target |
Buparlisib |
NCT02303041 |
|
Efficacy Study, Open Label |
PIK3 |
Protein kinase C (PKC) |
Clinical trial identifier no. |
Phase |
Study design |
Target |
Ingenol Mebutate (PEP005) |
NCT00108121 |
Phase II |
Safety/Efficacy/Double-blind |
Protein kinase C (PKC) |
Table 3. Non-FDA Approved Protein Kinase C[18].
Buparlisib: A specific oral inhibitor of the pan class I phosphatidylinositol 3-kinase (PI3K) family of lipid kinases with potential antineoplastic activity. Buparlisib specifically inhibits class I PIK3 in the PI3K/AKT kinase (or protein kinase B) signaling pathway in an Adenosine triphosphate (ATP)-competitive manner, thereby inhibiting the production of the secondary messenger phosphatidylinositol-3, 4, 5-trisphosphate and activation of the PI3K signaling pathway. This may result in inhibition of tumor cell growth and survival in susceptible tumor cell populations. Activation of the PI3K signaling pathway is frequently associated with tumorigenesis. Dysregulated PI3K signaling may contribute to tumor resistance to a variety of antineoplastic agents.
Protein kinase C (PKC)
Non-FDA approved protein kinase C (PKC): This non-FDA approved protein kinase includes:
Ingenol mebutate (PEP005): A selective small-molecule activator of protein kinase C (PKC) isolated from the plant Euphorbia peplus with potential antineoplastic activity. Ingenol mebutate (I3A) activates various protein kinase C (PKC) isoforms, thereby inducing apoptosis in some tumor cells, including myeloid leukemia cells, melanoma cells, and basal cell carcinoma cells. The PKC family consists of signaling isoenzymes that regulate many cell processes including proliferation, differentiation, and apoptosis.
Conclusion
Basal cell carcinoma is a non-melanoma type of skin cancer. It is formed in the lower part of the epidermis. The incidences of BCC are higher in white people. Australia is the geographical region where most cases of BCC are diagnosed. It generally occurs in the adults after the age of 40 years. Men have predominance over women in incidences of BCC. Immunotherapy, as well as molecular targeted therapy, has proven to be effective in the treatment of BCC. Imiquimod, sonidegib and vismodegib are the only FDA approved immunotherapeutic present for superficial BCC. Our success in treating BCC is increasing and advancing with the knowledge of the function of the immune system. Immunotherapy has shown a promising development in the past few years. The recent activities have increased our understanding of the tumor microenvironment, various immunotherapeutic modalities or combination therapy (like chemotherapy with immunotherapy). Additionally, the effects of such combination treatment modalities are still at exploratory phase. Proper preclinical and clinical designs are the important pillars in understanding the future of immunotherapy in treating cancer patients.
References
- What are the key statistics about basal and squamous cell skin cancers? 2013 October 21 [updated 2014 February 20; cited 2014 December 24].
- Rogers, Howard. “Your new study of nonmelanoma skin cancers.” Email to The Skin Cancer Foundation. March 31, 2010) (Skin Cancer Facts; Skin Cancer Foundation. Cited on 2015Jan16.
- Gloster HM Jr, Neal K (2006) Skin cancer in skin of color. J Am Acad Dermatol 55: 741-760. [Crossref]
- Basal cell carcinoma. 2014 [cited 2014 December 24]. Available from: http://www.clinuvel.com/en/skin-science/skin-conditions/skin-cancer/basal-cell-carcinoma-skin-cancer.
- Mohan SV, Chang ALS. Advanced basal cell carcinoma: epidemiology and therapeutic innovations. Curr Dermatol Rep 2014; 3(1): 40-45. Published online Feb 9, 2014.
- Diseases and ConditionsBasal cell carcinoma. 2014 [Updated 2013Sep. 07; cited 2014].
- Reifenberger J (2007) [Basal cell carcinoma. Molecular genetics and unusual clinical features]. Hautarzt 58: 406-411. [Crossref]
- Caro I, Low JA (2010) The role of the hedgehog signaling pathway in the development of basal cell carcinoma and opportunities for treatment. Clin Cancer Res 16: 3335-3339. [Crossref]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, et al. (1998) Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391: 90-92. [Crossref]
- Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH Jr, et al. (1997) Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276: 817-821. [Crossref]
- Kinzler KW, Vogelstein B (1997) Cancer-susceptibility genes. Gatekeepers and caretakers. Nature 386: 761, 763. [Crossref]
- Morlon A, Munnich A, Smahi A (2005) TAB2, TRAF6 and TAK1 are involved in NF-kappaB activation induced by the TNF-receptor, Edar and its adaptator Edaradd. Hum Mol Genet 14: 3751-3757. [Crossref]
- Tilli CMLJ, Steensel MAMV, Krekels GAM et al (2005) Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol 152: 1108–1124. [Crossref]
- FDA Approved label Imiquimod (Aldara) Manufactured by 3M Health Care Limited, England. Last updated on 2010.
15.FDA Approved label vismodegib (Erivedge™) Manufactured by Patheon, Inc. Mississauga, Canada Last updated on 2012.
16.Buparlisib in Treating Patients with Advanced or Metastatic Basal Cell Carcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2014 Dec 29
17.Peplin; Peplin. Study to Determine the Safety of Two Applications of PEP005 Topical Gel to Nodular Basal Cell Carcinoma. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2014 Dec 29.

