Introduction: There is no effective therapy for amyotrophic lateral sclerosis (ALS). Safety and feasibility of intrathecal autologous stem cell injections in ALS patients is described.
Methods: Fifteen ALS patients were enrolled. ALS functional rating scale revised (ALSFRS-R) was performed. They received subcutaneous filgrastim (600 mg) for 3 days. Then, CD34+ stem cells (150 ml) were bone marrow obtained and intrathechally injected. Patients were followed up for 1-year.
Results: Eight females and 7 males were included (mean 47.6 years). Interval between onset and stem cell injection was 23.8 months. Mean ALSFRS-R at inclusion and six months was 33 and 32, respectively. Four patients died, mortality was associated to scores lower than 30 points at inclusion.
Discussion: Intrathecal stem cell injection is safe, and well tolerated in ALS patients. Further studies with a greater number of patients are necessary to define the usefulness of intrathecal stem cell therapy in ALS patients.
Amyotrophic lateral sclerosis (ALS), safety, stem cells, bone marrow, hematopoietic stem cell transplantation
Abbreviations: ALS: Amyotrophic lateral sclerosis; ALSFRS-r: Amyotrophic lateral sclerosis functional rating scale revised; BM: Bone marrow; CBC: Complete blood cell count; CSF: Cerebrospinal fluid; CTCAE: Common terminology criteria for adverse events; FVC: Forced vital capacity; IT: Intrathecal; LMN: Lower motor neuron; MMSE: Mini mental state examination; MN: Motor neuron; MNC: Mononuclear cells; MRI: Magnetic resonance imaging; OD: onset to diagnosis; OI: Onset to inclusion; UMN: Upper motor neuron
Amyotrophic lateral sclerosis (ALS) is a late-onset neurodegenerative disorder characterized by rapid deterioration and selective death of motor neurons (MNs) in the cerebral cortex, brain stem, and spinal cord [1-3]. Clinical features are attributable to the superimposition of motor deficits occurring in the upper motor neuron (UMN) and lower motor neuron (LMN). Motor phenotypes are highly heterogeneous and are defined by: 1) the body region of onset; 2) the relative mix of UMN and LMN involvement; and 3) the rate of progression [4]. According to different series, mean survival of ALS patients ranges from 15.7 months to 47 months after presentation [5,6].
There is no effective therapy for ALS patients. Riluzole is the only medication approved by the FDA. However, this drug only slightly delays disease progression [7]. Stem cell therapy is considered another method for treating neurodegenerative disorders, including ALS. Stem cell infusion could be a potential therapeutic strategy, based not only on cell replacement but also on the modification of the extracellular motor neuronal environment, through trophic and neuroprotective effects [8]. A variety of cell sources have been considered for cell therapy, adult and embryonic stem cells can differentiate into several specialized lineages [8-10]. Adult stem cells are found within many tissues of the body. These cells can be more easily isolated from the peripheral blood or bone marrow (BM), and they remain the main source of stem cells capable of differentiation into several types, such as osteoblasts, chondrocytes, endothelial cells, glia, neurons, and skeletal and cardiac myocytes [8-19]. Several adult stem cells are available including mesenchymal stromal cells (MSCs) [11] and hematopoietic stem cells (HSCs) [12,16,17] derived from several sources, including BM, adipose tissue and umbilical cord blood.
Clinical studies using stem cells in humans have been described in Huntington’s disease, Parkinson’s disease, spinal cord injury, stroke, and Batten’s disease [18-23]. Current clinical trials are based on two main administration strategies: the systemic [24] and local approaches [25-28] Stem cell delivery into the spinal cord and the placement into the frontal motor cortex have been described and reported as safe methods [23, 26,27] After introducing stem cells into the subarachnoid space of the spinal cord, these cells can be transported by cerebrospinal fluid (CSF) and delivered more accurately to the injured areas than is expected by using the systemic route [25]. Furthermore a theoretical framework exists suggesting that cells injected directly into the subarachnoid space can be a safe approach [29-31]. There are several published studies regarding the safety of subarachnoid cell placement by intrathecal (IT) injection, with various cell types, methods of procurement, doses administered and acceptable adverse rates [30,31].
It is important to note that there is successful experience in hematopoietic BM transplantation through the use of granulocyte colony-stimulating factor (G-CSF) in order to increase the number of stem cells in BM and peripheral blood. Several types of progenitor cells can be obtained from human marrow, including MSCs, endothelial and very small embryonic stem cells [30,31]. Nowadays, mobilized peripheral blood is the more attractive source of cells for allogeneic and autologous stem cell transplantation, however, G-CSF stimulated BM has several advantages in the setting of cell therapy, higher concentration of different cells can be obtained in a lower volume from the BM, and it has been reported that MSCs among others, increase their numbers in situ after G-CSF administration without mobilization to the peripheral blood [31].
To assess both safety and feasibility, we have performed and uncontrolled, open-label non-randomized clinical trial in ALS patients. The scientific rationale of this study was aimed to improve motor neuron function in ALS subjects by means of intratechal BM nucleated and HSCs infusion.
Study subjects
This study was designed as a prospective, open-label, non-randomized clinical trial in a single center for ALS patients 18 to 70 years of age. All patients were recruited and evaluated for their eligibility at the neurology service of the “Dr. José E. González” University Hospital of the Universidad Autónoma de Nuevo León, (UANL) in Monterrey, México, between June 2012 and March 2013. The Institutional Review Board approved the protocol. Procedure risks were explained in detail to all patients enrolled and were required to sign an informed consent form. This trial was registered in Clinical Trials.gov (registration number NCT 01933321). A board certified and trained neurologist conducted examinations to confirm the diagnosis of ALS, according to the El Escorial clinical and neurophysiologic criteria [32,33]. Patients with a current or past history of neurological disease other than ALS and those enrolled in other clinical trials were excluded. The inclusion criteria were: (a) confirmed ALS according to the El Escorial clinical and neurophysiologic criteria; (b) no structural damage to the brain or spinal cord on cervical and cranial magnetic resonance imaging (MRI); (c) pulmonary function test showing a forced vital capacity (FVC) of at least 30; and (d) appropriate nutritional status, with a body mass index of at least 19. The exclusion criteria were: (a) severe bulbar affection; (b) inadequate nutritional status or a body mass index lower than 18; (c) tracheostomy; (d) presence of systemic disorders, such as malignant neoplasm, cardiovascular disease, previous stroke, or coagulation abnormalities; and (e) evidence of cervical spondylotic myelopathy or structural abnormalities on MRI. An electrocardiogram, complete blood count, routine coagulation studies and biochemical profile were performed. Infectious diseases such as viral hepatitis B and C, and HIV were also ruled out by standard methods.
The neurological examination consisted of testing for muscle tone, stretch reflexes, pathological reflexes, and the Medical Research Council scale for grading muscle power and strength [34]. The ALS Functional Rating Scale Revised (ALSFRS-R) [35], which is the most widely used and extensively validated global scale for assessing motor function in ALS, was used in our patients. This scale is weighted toward limb and bulbar function, and gives a total severity score out of 48 possible points. Patients with greater disability have a lower score. A mini mental state examination (MMSE) was performed for all patients. The entire clinical evaluation lasted 30-min and was performed at baseline at the first visit, followed by evaluations at 1,2,3, 6, and 12 months after transplantation.
Stimulation and harvesting of BM cells
After their informed consent was obtained, included patients received a subcutaneous daily dose of 10 mg/ kg of filgrastim (Neupogen, Basel, Switzerland) for a period of three days on an outpatient basis [36,37]. The day following the final dose of G-CSF, patients were admitted to hospital, and their white blood cell count was measured. After admission, BM collection was carried out. The patient underwent light sedation with IV midazolam (5 mg) and 1% xylocaine as local anesthesia. After asepsis of posterior iliac crest and the patient in prone position, Jamshidi needles were inserted on both iliac crests and 150 ml of BM as total volume was obtained. BM was collected in a mixture of ACD-A anticoagulant and 1000 U heparin. To remove micro aggregates, fat and bone fragments, a 170-micron filter in a laminar flow hood was used. An aliquot of BM was obtained and a complete blood cell count (CBC) was performed on a hematology analyzer Sysmex XT4000i and flow cytometry enumeration of CD34+, CD45+ plus viability with 7AAD (7-aminoactinomycin D) assessment were made using the single-platform counting on a flow cytometer (FACS Canto II Beckton Dickinson).
BM separation method of the mononuclear cells (MNC) was performed by centrifugation at 18° C in an open system and into the laminar flow hood. This procedure allowed us to obtain a product with a high concentration of MNC. Residual erythrocytes were removed with a lysing solution of ammonium chloride 1:10 for 5 minutes and subsequent washings with 0.9% saline; this washing procedure was repeated once to remove as much ammonium chloride as possible. The pellet was re-suspended in 10 ml saline solution. A 0.5-ml sample of this product was used to perform a CBC, bacterial cultures and flow cytometry for CD45+, CD34+ and viability determination.
The same day after BM was obtained nucleated and HSCs were delivered into the IT space. The patient was placed in lateral position and skin in the lumbar region was cleaned. The L3-L4 intervertebral space was located and 1% xylocaine (3 ml) was used as a local anesthetic. A number 22 Quincke needle was placed into the subarachnoid space and a sample of 10 ml CSF was extracted. Then, BM nucleated and HSCs (10 ml saline containing at least 5 million CD34+ HSCs) were infused into the subarachnoid space in about 5 minutes (Table 1), after cell infusion the needle was removed. All patients were kept under medical supervision until recovery from anesthesia. They were discharged in the same day of hospital admission, when they were fully awake and tolerating oral fluids. Patients were instructed to remain horizontally for at least 24 hours and to report if pain, fever, nausea or vomiting occurred.
Adverse events
All the ALS patients included in this study were followed for a year; the adverse events (AEs) were monitored from inclusion (baseline) up to one year after stem cells injection. During hospitalization, all AEs were recorded sequentially after every intervention, as described in the protocol: 1. Filgrastim injection; 2. MRI study; 3. BM aspiration under local anesthesia; 4. Lumbar puncture; and 5. Intrathecal injection. 6. Intravenous infusion. During follow-up presence of adverse events were registered according to the common terminology criteria for adverse events (CTCAE) that it is used to standardize the severity of the AEs (grades I–V) and to classify them appropriately [38].
Data analysis
Descriptive statistics were used for all variables. Normality was assessed with the Shapiro-Wilks test. The t-test was used for all variables as well as comparisons among ALS patients who survived or died during follow up. For multiple comparisons we used Bonferroni correction analysis. All statistical analyses were performed with the SPSS 22.0 software package (IBM, Chicago IL, USA).
Eight females and 7 males suffering from ALS were enrolled in the study. Mean age at inclusion was 47.4 years (range 33-67). Motor phenotypes at baseline were heterogeneous. In 8 patients LMN and UMN were equally affected, 5 patients presented with predominant LMN involvement and only 2 had a predominantly UMN phenotype (Table 1). There were no differences in outcome in relation to motor phenotypes. The mean interval between clinical onset to diagnosis (OD) was 13.9 months in all subjects (range 1-42) and the mean interval among onset to inclusion (OI) to treatment protocol was 27 months (range 12-57). The pulmonary function test at baseline showed a median forced vital capacity (FVC) of 3.38 ml (range 1.61-5.08) corresponding to mean of 84% of the FVC expected (range 73-96). The median baseline ALSFRS-R score in the whole group was 33.3 (Table 2). There were no abnormalities on laboratory at inclusion. No adverse events were observed during MRI. Back pain and pain in the posterior iliac crest were the only adverse events registered after lumbar puncture (LP) and BM extraction (CATCAE, grade I) which were controlled by analgesics; meningism, meningitis and headache due to LP or IT injections were not observed during follow up in these patients.
We injected 5.0 × 106 BM CD34+ HSCs in 10 ml of saline solution by IT route. We controlled that the infused sample was free of red blood cells. The viability of stem cells of each ALS subject included in our research protocol was confirmed. These BM stem cells were viable for up to 4 h after dilution. In our protocol the time elapsed between the enrichment of the sample and the IT injection was less than 1 h.
Increments in ALSFRS-R scores were observed in 13 out of 15 patients at one month after IT injections. Follow-up scores showed a mean of 37.07, 34.7, 32.2 and 29.2 at one month, 3 months, 6 months and 12 months after BM nucleated and hematopoietic stem cell injections, respectively (Table 2). Although there was an increment in the clinical scale during the first three months after treatment, there were no statistically significant differences among scores (Figure 1 and Table 2).
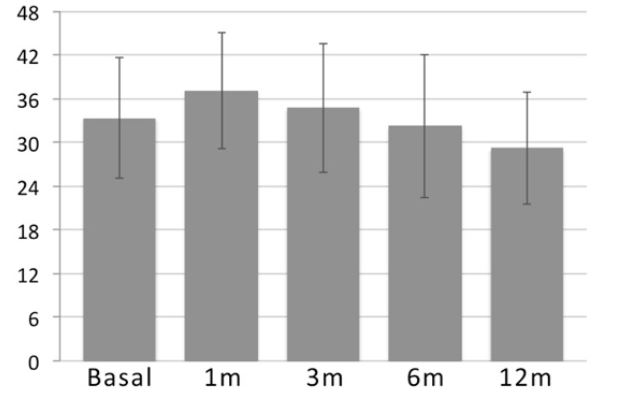
Figure 1. ALSFRS-r scores (mean values) follow up from basal, 1month, 3 months, 6 months and 12 months.
Mortality occurred in 4 cases, due to respiratory insufficiency in two, pneumonia in one and myocardial infarction in one patient. Deaths occurred at 3, 6, 8 and 10 months after the therapeutic procedure (mean 6.7) mean survival rate in these patients was 36 months (range 22-60). In 3 cases, information about survival and ALSFRS-R was obtained by phone in the 12 months evaluation (Table 2). Among dead patients, 3 had basal ALSFRS-R less than 30 points (20, 22 and 29 points). Surviving patients had a shorter OI interval of 19.2 months (range 13-49) in comparison to dead ALS patients for whom the OI interval was 29.2 months (range 12-57). Cytochemical evaluations in CSF including protein and cells were normal in all 15 ALS patients. CSF cultures were negative.
Table 1. Demographic characteristics in ALS patients
OD: Onset to diagnosis, OI: Onset to Inclusion, FVC: Forced Vital Capacity, LMN: Lower Motor Neuron,
MAI: Months After Injection, *Information obtained by phone, + Death
There are no effective therapeutic interventions for ALS. Delivery of BM nucleated cells to achieve neuroprotective and neurorestorative effects is an attractive goal [39], since BM provides several kind of cells, as previously noted, with regenerative potential besides CD34+ stem cells [28,30,31]. Other advantages of this kind of source for stem cells include fewer days of G-CSF stimulation, no need for central catheter placement or apheresis procedures, and therefore low complication rate in this kind of very sick patient [31]. We treated 15 ALS patients by injecting IT stem cells aimed to improve MN function. Human intrathecal transplantation of peripheral blood stem cells have been previously attempted in patients with ALS25 as well as BM derived stem cells in other neurological disorders like cerebral palsy, stroke, autism and spinal cord injury, with promising results and no relevant complications [8,18,23,30,31]. Although Janson in 2001 described IT transplantation of human stem cells obtained from peripheral blood, [25] to our knowledge this is the first study that includes IT G-CSF stimulated BM cells for the treatment of patients suffering from ALS, and the more important goal of this study was to clarify the safety of IT administration of BM cells without the use of apheresis, selection, or ex-vivo cell-expansion methods, using a simplified and affordable procedure in an ambulatory setting. A previous study [40] describes an optimum number of CD-34+ autologous stem cell transplantation (5.0 × 106) therefore we decided to infused at least 5 million CD34+ HSCs by IT route in our ALS subjects.
2021 Copyright OAT. All rights reserv
Motor phenotypes in the study group were highly heterogeneous as Ravitz and La Spada [4] recently analyzed. Heterogeneity of motor phenotypes is a clinically well-recognized fundamental aspect of ALS [2,4,6]. In the present series, 8 ALS patients showed LMN–UMN equally affected and 5 cases had predominantly LMN. The mean age at inclusion (47.4 years) was lower than the age reported in different ethnic populations [6]. It has been described that ALS age of onset in northern Sweden ranges from 60 to 64 years in males and from 70 to 74 years in females; in Ireland was 66 years and in Scotland the age of ALS onset was 65.2 years in males and 67.2 years in females [6]. Demographic as well as biologic factors could certainly determine the early age of onset of ALS in Hispanic population. The clinical onset to ALS diagnosis in this study had a mean of 13.9 months. This OD interval is in agreement with previous reports, however in patients younger than 40 years the OD interval was longer (case 3, 8 and 15) than in those older than 40 years (Table 1). The ALSFRS-R mean score presented a small increment in 13 out of 15 patients at one month after stem cells injections. This non statistically significant increase in the scale may possibly be related to filgastrim stimulation or growth factors released by the stem cells injected, however the placebo effect cannot be dismissed. Average declines of -1.1 ALSFRS-R score per month (-13.32 per year) have been reported [39,41]. In the present study a total decrement in ALSFRS-R mean score was of 4 points at one year in comparison to the mean score at inclusion. We consider that ALS patients enrolled in this study showed a slower progression of their disease during a year of follow up. The heterogeneous presentations of ALS emphasize the need for subject-specific baseline data [39]. This study has several limitations, we acknowledge that it was not powered to determine efficacy and there was no control arm, however IT stem cell injections appeared to be a safe and feasible procedure in ALS subjects. Another advantage of this study is its prospective nature and the relatively large group of patients studied. Previous reports related to stem cell transplantation in ALS patients have included a smaller number of subjects using the systemic, IT or direct frontal motor cortices route even by using systemic [16,17,24] or local routes [23,26,28,39] including stem cell transplantation in spinal cord [23] or frontal motor cortices [26].
Mortality occurred between 22 and 60 months with a mean survival of 36 months. Among survivors mean OI interval was considerably shorter (19.2 months) than in fatal ALS cases (29.2 months). This finding strongly suggests that only subjects early in the disease course may experience clinical benefits after stem cells injection. According to different series, survival of ALS patients ranges from 15.7 to 47 months after presentation with a median of 29.1 months [5,6]. Among dead patients, 3 had basal ALSFRS-R less than 30 points (20, 22 and 29 points) at the time of inclusion to this protocol. This observation may be important and suggests that a score lower than 30 points in the scale may be considered an exclusion criterion in clinical trials. Life expectancy in the present series was not modified in deceased ALS cases after IT stem cell injection.
Despite the limitations of the present study, we were able to identify potential clinical indicators of outcome for this approach. These indicators include: ALSFRS-R score lower than 30 points and longer OI interval (clinical presentation to stem cell treatment). Mortality was not correlated with FVC, clinical onset (spinal or bulbar) and UMN or LMN involvement. However, ALS with severe bulbar affection was an exclusion criterion in this trial.
IT-BM nucleated and stem cells injection is a safe, feasible and well-tolerated procedure with minimal and transient adverse events. Although slight improvement was observed in ALSFRS-R score during the first trimester after stem cells injection, the placebo effect cannot be dismissed. Several questions remain to be answered such as: is the BM the best source of cells for this kind of therapy? How many cells are needed? Which cell is the most important? Is the intrathecal route of cell-delivery useful? Controlled clinical trials adequately powered and including ALS patients at homogeneous clinical stages should be conducted to definitively establish efficacy of this procedure.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
- Mills KR (2003) The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain 126: 2558-2566. [Crossref]
- Toft MH, Gredal O, Pakkenberg B (2005) The size distribution of neurons in the motor cortex in amyotrophic lateral sclerosis. J Anat 207: 399-407. [Crossref]
- Ringel SP, Murphy JR, Alderson MK, Bryan W, England JD, et al. (1993) The natural history of amyotrophic lateral sclerosis. Neurology 43: 1316-1322. [Crossref]
- Ravits JM, La Spada AR (2009) ALS motor phenotype heterogeneity, focality, and spread. Deconstructing motor neuron degeneration. Neurology 73: 805-811.
- Zoccolella S, Beghi E, Palagano G (2008) Analysis of survival and prognostic factors in amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 79: 33-37.
- Martínez HR, Molina-López JF, Cantú-Martínez L, González-Garza MT, Moreno-Cuevas JE, et al. (2011) Survival and clinical features in Hispanic amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler 12: 199-205. [Crossref]
- Lomen-Hoerth C (2008) Amyotrophic lateral sclerosis from bench to bedside. Semin Neurol 28: 205-211. [Crossref]
- Kim SU, de Vellis J (2009) Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res 87: 2183-2200. [Crossref]
- Cho KJ, Trzaska T, Greco SJ, McArdle J, Wang FS, Ye JH, et al. (2005) Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1a. Stem Cells 23: 383-391.
- Levy YS, Stroomza M, Melamed E, Offen D (2004) Embryonic and adult stem cells as a source for cell therapy in Parkinson's disease. J Mol Neurosci 24: 353-386. [Crossref]
- Long X, Olszewski M, Huang W, Kletzel M (2005) Neural cell differentiation in vitro from adult human bone marrow mesenchymal stem cells. Stem Cells Dev 14: 65-69. [Crossref]
- Yamamoto R, Ishikawa M, Tanaka N, Kamei N, Nakanishi K, et al. (2005) CD133+ cells from human peripheral blood promote corticospinal axon regeneration. Neuroreport 19: 799-803.
- Aejaz HM, Aleem AK, Parveen N, Khaja MN, Narusu ML, et al. (2007) Stem cell therapy-present status. Transplant Proc 39: 694-699. [Crossref]
- Choumerianou DM, Dimitriou H, Kalmanti M (2008) Stem cells: promises versus limitations. Tissue Eng Part B Rev 14: 53-60. [Crossref]
- Delcroix GJ, Schiller PC, Benoit JP, Montero-Menei CN (2010) Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials 31: 2105-2120. [Crossref]
- Cashman N, Tan LY, Krieger C, Mädler B, Mackay A, Mackenzie I, et al. (2008) Pilot study of granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells in amyotrophic lateral sclerosis (ALS). Muscle Nerve 37: 620-625.
- Appel SH, Engelhardt JI, Henkel JS, Siklos L, Beers DR, et al. (2008) Hematopoietic stem cell transplantation in patients with sporadic amyotrophic lateral sclerosis. Neurology 71: 1326-1334. [Crossref]
- Yoo J, Kim HS, Hwang DY (2013) Stem cells as promising therapeutic options for neurological disorders. J Cell Biochem 114: 743-753. [Crossref]
- Isacson O (2003) The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurol 2: 417-424. [Crossref]
- Movigilia GA, Fernandez Vina R, Brizuela JA, Saslavski J, Vrsalovic F, Varela G, et al. (2006) Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy 8: 202-209.
- Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, et al. (2006) Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med 29: 191-203. [Crossref]
- Schwarz SC, Schwarz J (2010) Translation of stem cell therapy for neurological diseases. Transl Res 156: 155-160. [Crossref]
- Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, et al. (2010) Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol 223: 229-237. [Crossref]
- Silani V, Cova L, Corbo M, Ciammola A, Polli E (2004) Stem-cell therapy for amyotrophic lateral sclerosis. Lancet 364: 200-202. [Crossref]
- Janson CG, Ramesh TM, During MJ, Leone P, Heywood J (2001) Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. J Hematother Stem Cell Res 10: 913-915.
- Martinez HR, Gonzalez-Garza MT, Moreno-Cuevas J, Caro E, Gutierrez-Jimenez E, et al. (2009) Stem-cell transplantation into the frontal motor cortex in amyotrophic lateral sclerosis patients. Cytotherapy. 11: 26-34.
- Martínez HR, Molina-Lopez JF, Gonzalez-Garza MT, Moreno-Cuevas JE, Caro-Osorio E, et al. (2012) Stem cel transplantation in amyotrophic lateral sclerosis patients. Methodological approach, safety and feasibility. Cell Transplantation 21: 1899-2012.
- Deda H, Inci MC, Kürekçi AE, Sav A, Kayihan K, et al. (2009) Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy 11: 18-25. [Crossref]
- Bartley J, Carroll JE (2003) Stem cell therapy for cerebral palsy. Expert Opin Biol Ther 3: 541-549. [Crossref]
- Sharma A, Gokulchandran N, Chopra G, Kulkarni P, Lohia M, Badhe P et al. (2012) Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transplant 21(Suppl 1): S79-S80.
- Mancias-Guerra C, Marroquin-Escalante AR, Gonzalez-Llano O, Villarreal-Martinez L, Jaime-Perez JC, et al. (2014) Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: an open-label phase I trial. Cytotherapy 16: 810-820.
- Brooks BR, Miller RG, Swash M, Munsat TL (2000) Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyothrop Lateral Scler Other Motor Neuron Disord 1: 293-299.
- Miller RG, Munsat TL, Swash M, Brooks BR (1999) Consensus guidelines for the design and implementation of clinical trials in ALS. World Federation of Neurology committee on Research. J Neurol Sci 169: 2-12. [Crossref]
- John J (1984) Grading of muscle power: comparison of MRC and analogue scales by physiotherapists. Medical Research Council. Int J Rehabil Res 7: 173-181. [Crossref]
- Kaufmann P, Levy G, Thompson JL, Delbene ML, Battista V, et al. (2005) The ALSFRSr predicts survival time in an ALS clinic population. Neurology 64: 38-43. [Crossref]
- Hashimoto S, Itoh M, Nishimura M, Asai T (2002) Effect of filgrastim administration for steady-state mobilization of peripheral blood stem cells. Ther Apher 6: 431-436. [Crossref]
- Kröger N, Renges H, Krüger W, Gutensohn K, Löliger C, Carrero I, et al. (2000) A randomized comparison of once versus twice daily recombinant human granulocyte colony stimulating factor (filgrastim) for stem cell mobilization in healthy donors for allogeneic transplantation. Br J Haematol 111: 761-765.
- National Cancer Institute. U.S. National Institutes for Health; [updated 2010 Aug 12; cited 2010 Aug 31]. Available from http://ctep.cancer.gov
- Riley J, Glass J, Feldman E, Polak M, Bordeau J, et al. (2014) Intraspinal Stem Cell Transplantation in Amyotrophic Lateral Sclerosis: A Phase I Trial, Cervical Microinjection, and Final Surgical Safety Outcomes. Neurosurgery 74: 77-87.
- Jillella AP, Ustun C (2004) What is the optimum number of CD34+ peripheral blood stem cells for an autologous transplant? Stem Cells Dev 13: 598-606. [Crossref]
- Healy BC, Schoenfeld D (2012) Comparison of analysis approaches for phase III clinical trials in amyotrophic lateral sclerosis. Muscle Nerve 46: 506-511. [Crossref]

