Background: Pure laparoscopic liver resection is a less invasive procedure than conventional liver resection for hepatic lesions. A laparoscopic approach offers the advantage of Intra-operative laparoscopic ultrasonography (IOLUS), providing better establishment of the number and location of liver tumors, as well as the condition of the liver. The aim of the study is to assess the feasibility, safety and efficacy of laparoscopic resection of hepatocellular carcinoma (HCC) in patients with liver cirrhosis.
Patients and methods: Between September 2010 and January 2014, five hundred patients with liver cirrhosis and HCC were referred from the HCC clinic at the National Hepatology and Tropical Medicine Research Institute (NHTMRI). 65 patients were considered suitable candidates for diagnostic laparoscopy. 26 patients with HCC who fulfill the criteria of laparoscopic non-anatomical liver resection. Operation times, the duration of hospital stay, and post procedural complications were recorded. A spiral CT scan one month postoperatively was an essential part of the follow-up protocol.
Results: Twenty six patients were managed entirely by laparoscopic liver resection. IOLUS examination identified previously unsuspected HCC in three patients which treated on the same session. The mean operation time was 120 ± 50 minutes; eight additional procedures were undertaken in six patients, cholecystectomy in six and adhesiolysis in two cases. Complete tumors were resected and which was subsequently documented using a spiral computed tomography (CT scan) one month after treatment. Histopathology confirmed that the entire tumour had been resected with a margin of safety.
Conclusion: We have proved that a laparoscopic resection has a role in the management of HCC and it is a safe and effective technique.
Laparoscopic hepatectomy, Hepatocellular carcinoma (HCC), Intraoperative laparoscopic ultrasound, liver cirrhosis, liver tumors, liver resection
Hepatocellular carcinoma (HCC), the most common primary liver cancer, occurs in 90% of the cases in patients with chronic liver disease (CLD) [1]. In recent years, its incidence has increased as consequence of chronic hepatitis C virus infections [2]. Therapeutic options with curative intent for small HCCs are now increasingly recognized [3]. The optimal treatment for hepatocellular carcinoma (HCC) is surgical resection. However, only a small percentage of patients are operative candidates. Surgical resection is generally considered an efficient treatment, but its use in the context of chronic liver disease (CLD), is limited because of significant morbidity, which could be at least in part, related to the laparotomy itself. Since the advent of laparoscopic cholecystectomy, laparoscopic surgery has become a popular surgical technique [4] and has been applied to solid organs. Initial laparoscopic liver procedures included biopsies [5], tumour staging [6], and the fenestration of nonparasitic liver cysts [7]. The improvement in surgical techniques and also, various devices have been designed and developed to minimize the blood loss during transaction of the liver tissue which has allowed the development of laparoscopic liver resection to gain popularity. Recent advances in laparoscopic ultrasound have greatly improved the accuracy in detecting intrahepatic HCC nodules, many of which were missed by computed tomography [8]. Also, it will help for accurate evaluation of hepatic cirrhosis status as well as the outer and safety margin of HCC during surgery.
The study carried out between "September 2010 to January 2014" at NHTMRI with the approval of our Institutional Research Ethics Board. Five hundred patients with HCC in cirrhotic livers were referred from the HCC clinic to surgery. Twenty six patients with HCC who fulfilled the criteria (Table 1) were submitted to laparoscopic liver resection. Twenty three were male and 3 female. The mean age was 57.4 ± 6.4 years. Patients were classified according their Child-Pugh score. Preoperative triphasic (arterial, portal and venous) spiral CT examinations through seven-millimeter slices were obtained throughout the liver in each patient represented in this group. The maximum diameter of each visible lesion was measured and the precise location of the lesion was determined with the segmental anatomic mapping system proposed by the Brisbane World Congress of IHPBA (International Hepato-Pancreato-Biliary Association), defined by the distribution of the hepatic and portal venous systems [9]. Laparoscopic exploration and Liver ultrasound was performed with a flexible linear 7.5-mHz IOLUS transducer (Courtesy of BK Medical, Copenhagen, Denmark). All ultrasound examinations were carried out and interpreted by a single surgeon and single radiologist. 26 patients underwent Laparoscopic liver resection. A spiral CT scan one month postoperatively was an essential part of the follow-up protocol as was the level of alpha feto-protein.
Table 1. Inclusion criteria
- Chronic liver disease with Child's A.
- Solitary tumor ≥ 5 cm in diameter without vascular invasion.
- Exophytic lesion or lesion located in the anterior and peripheral segments.
- Patients with American Society of Anesthesiologists (ASA) I, II, and III .
- Patients with Platelet count ≥ 80 x 1010
|
The procedure was performed with the patient under endotracheal general anaesthesia and placed in a supine position on operating table. The surgeon stood on the left side of the patient and the first assistant on the opposite side. The monitors was placed at the head of the table. Through a supra-umbilical incision, with caution not to injury any of porto-systemic collaterals. A carbon dioxide pneumoperitoneum was induced using a Veress needle. Laparoscopic exploration was then undertaken with a zero degree laparoscope. The liver was inspected and the condition evaluated. Another 10mm port was placed left to the beginning of the faliciform ligament for ease of screening the whole liver by a laparoscopic ultrasound probe. An additional 5 mm port was inserted depending upon the location of the HCC and other port were inserted as demand. Laparoscopic ultrasound was then carried out of the whole liver followed by the localization of the HCC (Figure 1).
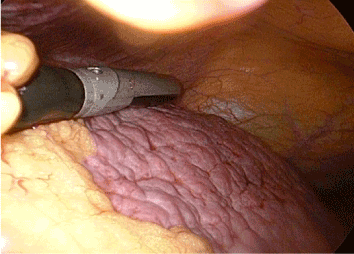
Figure 1. Intraoperative laparoscopic ultrasound liver scan
Surgical resection was recommended and proceeded with using a laparoscopic HabibTM 4X rediofrequency needle in those cases with subcapsular lesion, left segment II-III lesion, exophytic lesions segment III (Figures 2a and 2b), segment IVb (Figures 3a and 3b) and right segment V-VI (Figures 4a and 4b) hepatocellular carcinoma which were associated with a fair condition of the liver tissue. A drain was left in position, to be removed within 24-48 hours.
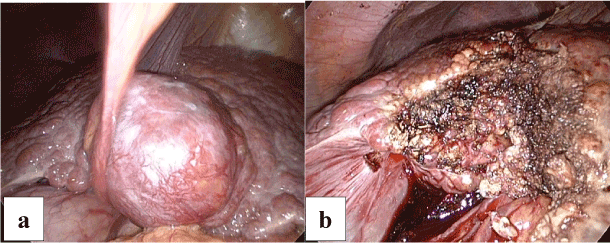
Figure 2. a. Laparoscopic view of Exophytic Hepatic Focal segment III.
b. After laparoscopic resection
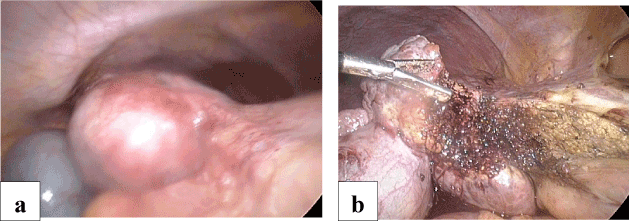
Figure 3. a. Laparoscopic view of Exophytic Hepatic Focal lesion segment IV.
b. After laparoscopic resection
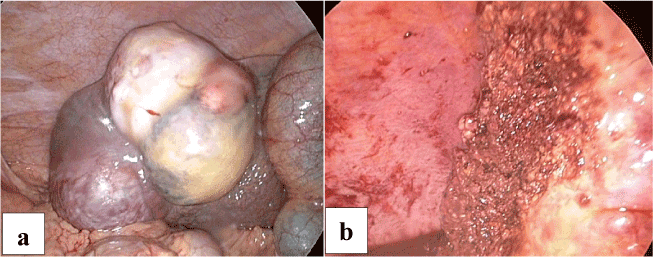
Figure 4. a. Laparoscopic view of Exophytic Hepatic Focal lesion segment V and VI.
b. After laparoscopic resection
A triphasic CT scan was carried out one month post-operatively and abdominal ultrasound and the alpha feto-protein (AFP) level were obtained every three months during follow up period. If the AFP became elevated, further imaging studies such as Triphasic CT scan was performed. Local recurrence was defined as tumor recurrence at the treated site. Whereas if a tumor appeared in different site within the hepatic parenchyma was defined as a new HCC. These recurrences or new HCCs were treated as best indicated depending on the individual circumstances.
Twenty six patients were included in this series. Twenty four patients were characterised as Child-Pugh class-A and two patients were early class-B. The IOLUS examination identified new HFLs in three patients which were treated accordingly. A total of 29 lesions were laparoscopically treated. Eight additional but unrelated procedures were performed in six patients: 6 cholecystectomies and 2 adhesiolysis. The lesions measured by preoperative ultrasound, the mean diameter of the tumors was 32 ± 15 mm. Laparoscopic ultrasound examination was attempted in all patients, but in two patients (7%) the presence of adhesions prevented intraoperative scanning of the liver so adhesiolysis was done and scanning completed. The mean operating time was 120 ± 50 minutes.
Histological examination of the resected specimen revealed hepatocellular carcinoma in 24 cases, focal nodular hyperplasia in one patient, and hepatocellular adenoma in one patient.
The mean hospital stay was 5.14 days. The incidence of postoperative complications in Laparoscopic resection was 15%, as the following, two cases developed postoperative ascites, one cases developed jaundice and one cases developed haematoma at operative surface. No recurrence was revealed during the 3 month post-operative fellow up using with triphasic CT and alpha FP in all patients. One case developed recurrence at the port site after 18 months follow up which was treated by local excision.
Laparoscopic liver resection was first reported in the early 1990s for partial resection of segment 6 for a 6 cm focal nodular hyperplasia and wedge resection of segment 5 for colorectal liver metastases [10]. Since then, the number of reported cases of laparoscopic liver resection has increased dramatically, especially over the past 5 years [11]. Although some surgeons remain sceptical about the oncological curative potential of laparoscopic surgery, and further evaluation of the long-term prognosis is required in order to justify the minimally invasive approach for hepatic malignancies, several specialized centers have expanded the indications for laparoscopic hepatectomy. These indications now include both benign and malignant tumors, including HCC [12] as well as resection of the posterosuperior segments, such as segment 8, 7, or 1 [13,14]. These operations, from wedge resection of the anterolateral segments to major hepatectomy, including even the most minor ones in cirrhotic patients, carry a high risk of complications. The aim of laparoscopic resection in cirrhotic patients was actually to reduce postoperative complications [15]. There are several advantage of laparoscopic surgery such as less pain, less wound, less bleeding, less adhesion. These advantages correspond with enhanced recovery after surgery, in order to reduce the possibility of inflammatory, humoral and immune response after surgery. Thus, laparoscopic liver resection can reduce the hospital stay [16]. In this series the mean hospital stay was 5.14 days. The most suitable candidates among HCC patients for laparoscopic hepatectomy are, in general, those with solitary lesions measuring 5 cm or less in diameter, which are located in the peripheral segments [17]. In this series we did follow those criteria. Several surgeons advocate laparoscopic resection for HCC, especially those in the cirrhotic liver, as the laparoscopic approach might offer improved results, as has been suggested by several reports [18]. However, one of the reasons why the laparoscopic surgery is not generally accepted is because of the oncology outcome. In many studies, they indicated that there are no differences in disease free survival (DFS) rate and overall survival (OS) rate between laparoscopic surgery and open surgery [19-22]. Other limitation of laparoscopic surgery are tumor cell seeding or tumor recurrence through port site. We have only one case of tumor seeding which treated by excision, however this can be prevented by careful manipulation of the tumor.
In conclusion, laparoscopic liver resection for HCC is feasible, safe and efficient for resection of HCC in patients with liver cirrhosis.
- Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362: 1907-1917. [Crossref]
- Deuffic S, Poynard T, Buffat L, Valleron AJ (1998) Trends in primary liver cancer. Lancet 351: 214-215. [Crossref]
- Yamamoto J, Okada S, Shimada K, Okusaka T, Yamasaki S, et al. (2001) Treatment strategy in hepatocellular carcinoma: Comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology 34: 707-717. [Crossref]
- Dubois F, Berthelot G, Levard H (1991) Laparoscopic cholecystectomy:historic perspective and personal experience. Surg Laparosc Endosc 1: 52-57. [Crossref]
- Falcone RE, Wanamaker SR, Barnes F, Baxter CG, Santanello SA (1993) Laparoscopic vs. open wedge biopsy of the liver. J Laparoendosc Surg 3: 325-329. [Crossref]
- John TG, Greig JD, Crosbie JL, Miles WF, Garden OJ (1994) Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound. Ann Surg 220: 711-719. [Crossref]
- Morino M, De Giuli M, Festa V, Garrone C (1994) Laparoscopic management of symptomatic nonparasitic cysts of the liver. Indications and results. Ann Surg 219: 157-164. [Crossref]
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, et al. (2003) Treatment of focal liver tumours with percutaneous radiofrequency ablation; complications encountered in a multicentre study. Radiology 226: 441-451. [Crossref]
- Douglas LC, Douglas PS, Richard JC, Bruce JD (2005) Laparoscopic nonanatomic hepatic resection employing the LigaSure device. JSLS 9: 35-38. [Crossref]
- Gagner MRM, Dubuc J (1992) “Laparoscopic partial hepatectomy for liver tumor [Abstract].” Surgical Endoscopy 6: 99.
- Nguyen KT, Gamblin TC, Geller DA (2009) “World review of laparoscopic liver resection-2,804 patients.” Ann Surg 250: 831–841. [Crossref]
- Koffron AJ, Auffenberg G, Kung R, Abecassis M (2007) “Evaluation of 300 minimally invasive liver resections at a single institution: less is more.” Ann Surg 246: 385–392. [Crossref]
- Cho JY, Han HS, Yoon YS, Shin SH (2008) “Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location.” Surgery 144: 32–38. [Crossref]
- Yoon YS, Han HS, Cho JY, Ahn KS (2009) “Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver.” Surg Endosc 24: 1630–1637. [Crossref]
2021 Copyright OAT. All rights reserv
- Bruix J, Castells A, Bosch J, Feu F, Fuster J, et al. (1996) Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology 111: 1018 –1022. [Crossref]
- Hoffmann H, Kettelhack C (2012) Fast-track surgery: conditions and challenges in postsurgical treatment: a review of elements of translational research in enhanced recovery after surgery. Eur Surg Res 49: 24-34. [Crossref]
- Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, et al. (2009) “The international position on laparoscopic liver surgery: the louisville statement.” Ann Surg 250: 825–830. [Crossref]
- Abdel-Atty MY, Farges O, Jagot P, Belghiti J (1999) Laparoscopy extends the indications for liver resection in patients with cirrhosis. Br J Surg 86: 1397-1400. [Crossref]
- Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, et al. (2005) Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg 189: 190-194. [Crossref]
- Kobayashi S, Nagano H, Marubashi S, Kawamoto K, Wada H, et al. (2013) Hepatectomy based on the tumor hemodynamics for hepatocellular carcinoma a comparison among the hybrid and pure laparoscopic procedures and open surgery. Surg Endosc 27: 610-617. [Crossref]
- Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, et al. (2010) Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc 24: 1170-1176. [Crossref]
- Belli G, Limongelli P, Fantini C, D'Agostino A, Cioffi L, et al. (2009) Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 96: 1041-1048. [Crossref]




