Radiofrequency cautery is widely used in minor surgery. However, it has not been considered to fit for major surgery. Herein, we report two cases of pneumothorax surgery using low-thermal coagulation by this general-purpose radiofrequency cautery device.
Case 1: A 47-year-old male underwent bullectomy with a general radiofrequency cautery. We used Elleman® Surgitron PellevéTM S5 (Elleman International, Inc., Hicksville, NY) with a ball-shaped electrode tip (5mm in diameter, 110mm in length, Erbe Elektromedizin GmbH, Tübingen, Germany) for surgical coagulation of emphysematous bullae and abrasion of apical parietal pleura.
Case 2: A 65-year-old female also underwent pneumothorax surgery with radiofrequency cautery. Pathological findings: Each coagulated bulla wall was degenerated, thickened, and turned into non-structured tissue by heat infiltration at low temperature of radiofrequency. This degeneration of collagen fibers was observed only within 2mm from the pleural surface.
Conclusion: Radiofrequency cautery made the bulla wall thickened and degenerated without damaging the lung parenchyma.
radiofrequency, surgery, pneumothorax, cautery, coagulation
Radiofrequency cautery is widely used in minor surgery, especially in the fields of plastic and cosmetic surgery, ophthalmology, head and neck surgery, gynecology, and urology. Compared with usual general electrocautery devices, radiofrequency cautery enables very delicate operation because the heat infiltration to the tissue could be more concentrated and the invasiveness to the patient could be minimized. However, for its “delicate” characteristics, radiofrequency cautery has not been considered to fit for major surgery.
There are various surgical procedures to deal with pulmonary bullae. The most standard method is surgical resection by endoscopic staplers. Contracting suture and ligation are also used. However, when the wall of the bulla is very thin, or the lung parenchyma is much emphysematous and fragile, stapling or suturing would be sometimes difficult because these procedures may break the weakened lung tissue, easily. Thus, staplers that are reinforced with polyglycolic acid sheet are now in the market and could be often used in such cases with fragile pleura. However, air leakage from the stapling line is still annoying problem. In these days, several electrosurgical devices that can cauterize the bulla wall and emphysematous tissue to get shrunk and thickened, without burnt, perforation, incision, carbonization, or destruction by low-thermal coagulation, are coming to the market. This is also called as “soft” coagulation. SOFT COAGTM mode of VIO® 300D (Erbe Elektromedizin GmbH, Tübingen, Germany), Medtronic® Monopolar SealerTM (Medtronic, Dublin, Ireland) are representative products. However, we could only a few studies describing about the usage of these coagulation device in pneumothorax surgery in the literature. Best to our knowledge, one English and two Japanese reports were found for Monopolar SealerTM [1,2-4] and only one Japanese report was found for SOFT COAGTM [5] Medtronic® Monopolar SealerTM EndoFB 3.0® Floating Ball, formerly-known as TissueLinkTM (unavailable now) or also known as Salient Surgical TechnologiesTM (unavailable now), is a radiofrequency device specializing in tissue sealing.
These products seem very useful but expensive to be introduced easily. For example, an AEXTM generator for Monopolar SealerTM costs approximately 34,000 USD and EndoFB 3.0® costs approximately 735 USD in Japan. The price of a set of VIO300D is approximately 70,660 USD. CovidienTM VLFT10 (Medtronic, Dublin, Ireland) that has been recently introduced to the Japanese market, equipped both soft coagulation and Ligasure® function, costs approximately 69,990 USD in Japan.
If general-purpose radiofrequency cautery could be used for ablation of pulmonary bullae, it would be easily introduced because the cost is cheaper than such devices. For example, general-purpose radiofrequency cautery devices such as Elleman® Surgitron PellevéTM S5 and Surgitron Dual (Elleman International, Inc., Hicksville, NY) costs 30,188 and 9,245 USD approximately for each, in Japan.
However, there is no previous report describing about a usage of general-purpose radiofrequency cautery device in pneumothorax surgery. Herein, we report two cases of pneumothorax surgery using this general-purpose radiofrequency cautery device for coagulation/ablation of pulmonary bullae by utilizing its low heat infiltration, with a concise review of mechanism of radiofrequency cautery and the literature.
A 47-year-old male presented with right chest and back pain suddenly onset during breakfast, was transferred to the emergency department of our hospital. He has a history of right pneumothorax 9 months before, and was treated only by tube thoracostomy. A chest radiograph demonstrated right pneumothorax, again. Thus, tube thoracostomy was performed, immediately. Air leakage was persisted for 10 days, and then bullectomy and pleurodesis by video-assisted thoracoscopic surgery (VATS) was undertaken on the 11th day from the admission. With written informed consent from the patient, we used Elleman® Surgitron PellevéTM S5 with a ball-shaped electrode tip (5mm in diameter, 110mm in length, Erbe Elektromedizin GmbH, Tübingen, Germany) for low-thermal coagulation of emphysematous bullae and abrasion of apical parietal pleura. Apical large bullae were cauterized with coagulation mode of Surgitron PellevéTM at the power of 20 watts (Figure 1A, 1B, 1C). The bulla wall got thickened then was resected with an endoscopic stapler (Figure 1D). Small bullae were cauterized with Surgitron PellevéTM also to get thickened, and left unresected (Figure 1E, 1F). Parietal pleura was also cauterized with cutting mode of Surgitron PellevéTM at the power of 10 watts to expose the muscular layer to get postoperative adherence for prevention of postoperative recurrent pneumothorax (Figure 1G). Each cauterized bulla was shrank and turned into thick white structure, without irrigation by saline. There was no burnt or no perforation macroscopically at the cauterized sites. The pleural cavity was irrigated with 400mL of 50% glucose solution to get entire pleural thickness. The operation time was 1:22 and the blood loss was 50g. The postoperative course was uneventful. The chest tube was removed on the day 1 and the patient was discharged on the day 3. He is well without recurrent disease seven months after the surgery.
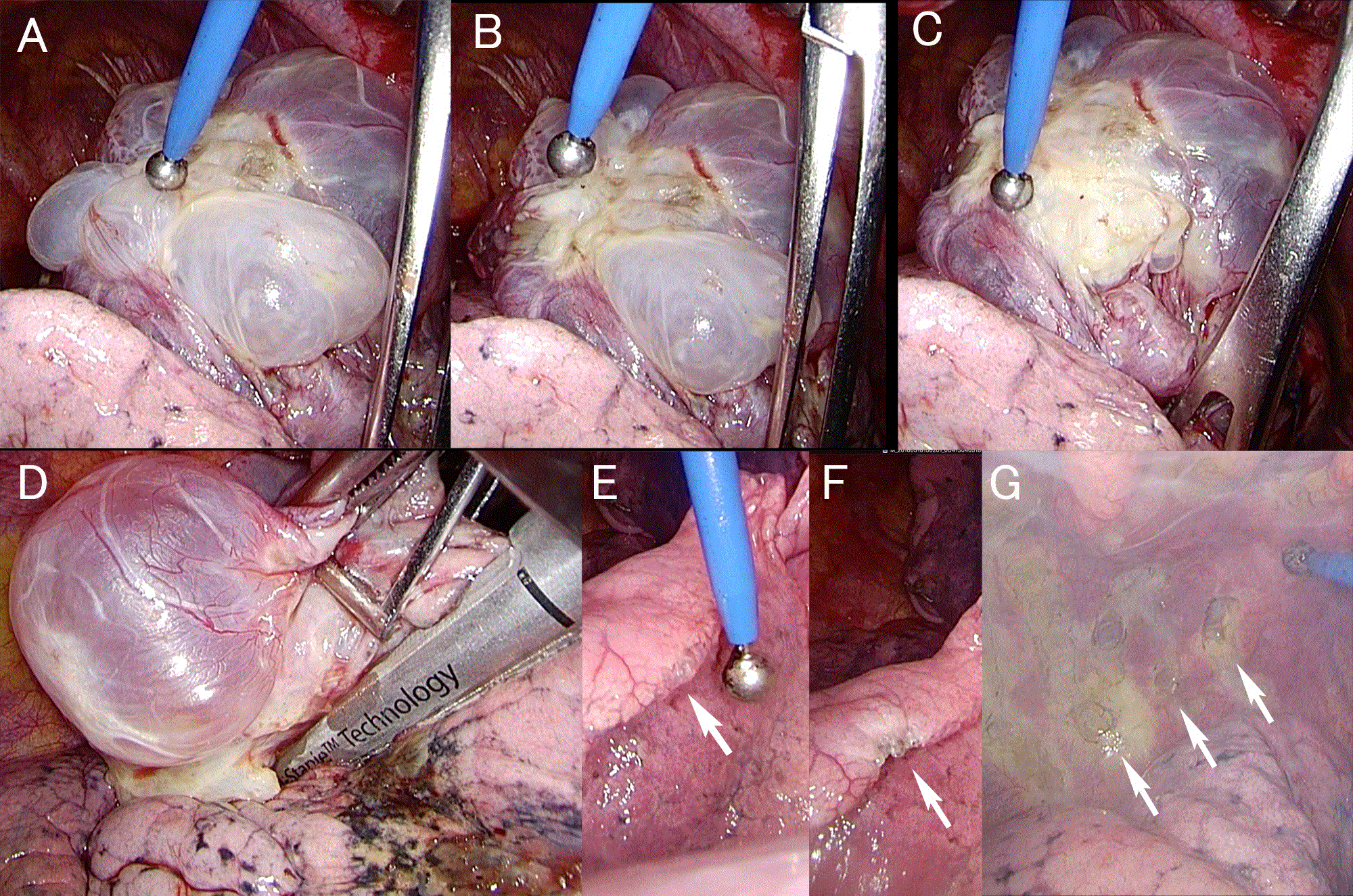
Figure 1. A. Apical large bullae were cauterized with coagulation mode of Surgitron PellevéTM at the power of 20 watts. B. The bulla wall is shrunk and gets thickened. C. The visceral pleura along the stapling line get thickened enough to apply a endoscoipic stapler. D. The apical bulla is resected by a stapler. E. A small bulla at the lober edge (arrow). F. A cauterized small bulla left unresected at the lober edge (arrow). G. Scarred parietal pleura by cutting mode of Surgitron PellevéTM at the power of 10 watts to expose the muscular layer to get postoperative adherence for prevention of postoperative recurrent pneumothorax (arrows).
A 65-year-old female with a history of surgically-treated left pneumothorax 13 years before, presented with gradually-worsening dyspnea for three months. She has also been diagnosed with pseudoxanthoma elasticum 14 years ago. She visited a clinic near-by and a chest radiograph showed right pneumothorax. She transferred to our hospital and underwent tube thoracostomy, immediately. Chest computed tomography (CT) scans demonstrated multiple bullae sporadically in the lung parenchyma and the visceral pleura. The air leak was intermittently observed and she underwent bullectomy and pleurodesis via VATS on the 9th day from the admission, with written informed consent from the patient for the intraoperative use of radiofrequency device. Fifteen small bullae and blebs that were less than 1.0 cm in diameter, were cauterized with coagulation mode of Surgitron PellevéTM at the power of 10 watts, until they turned into whitish thickened structure, then left unresected. Two large bullae of 2.0 cm and 3.0 cm in diameters were cauterized along the resection line, then cut with an endoscopic stapler. Neither carbonization nor perforation was observed at every coagulated site. The operation time was 2:11 and the blood loss was 85g. The postoperative course was uneventful. The chest tube was removed on the Day 2 and the patient was discharged on the Day 5. Seven months has been passed and she is well without recurrent disease.
Pathological findings of the bulla coagulated by radiofrequency cautery were shown in Figure 2. The coagulated bulla wall was degenerated, thickened, and turned into non-structured tissue by heat infiltration at low temperature of radiofrequency. Any inflammatory change responding to this degeneration was merely observed. A thin fibrin-like structure were covering the surface of the parietal pleura, seemed as a change that was accompanied by low-thermal coagulation. This degeneration of collagen fibers was observed only within 2mm from the pleural surface. Any pathological evidence of heat infiltration into the lung parenchyma was not demonstrated.
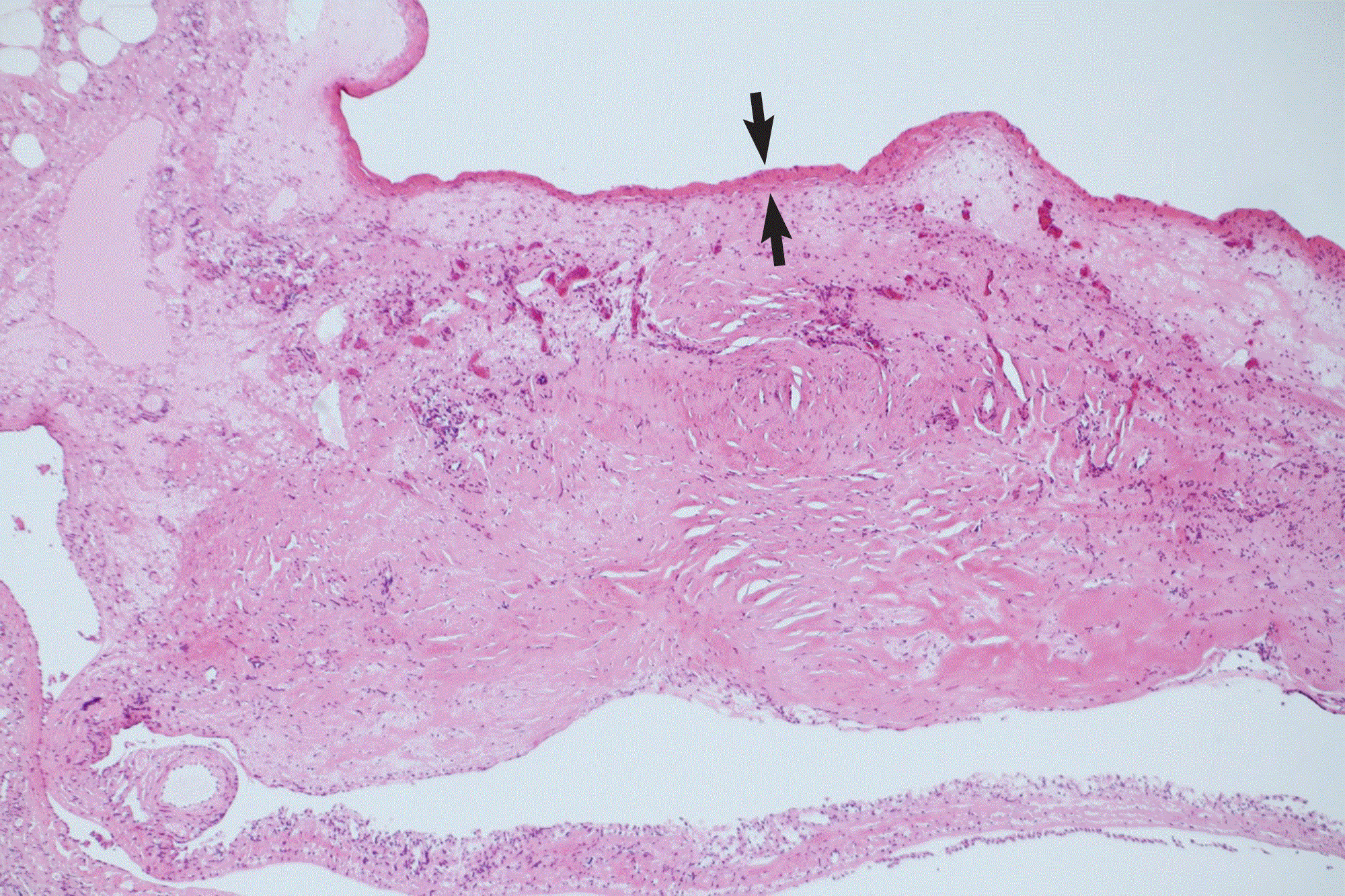
Figure 2. HE stain of coagulated bulla at low power (x40) demonstrates that degenerated, thickened, and non-structured tissue by heat infiltration at low temperature of radiofrequency. A thin fibrin-like structure were covering the surface of the parietal pleura, seems as a change that was accompanied by low thermal coagulation (black arrows). This degeneration of collagen fibers is observed only within 2mm from the pleural surface. Any pathological evidence of heat infiltration into the lung parenchyma is not demonstrated.
Generally, usual surgical electrocautery devices use electric current of around 300 - 400kHz. Frequencies between 300kHz and 3MHz are called as medium frequency and those between 3MHz and 30MHz are high frequency. Thus, common surgical electrocautery devices for general purpose, including Erbe’s VIO® that uses 350kHz, are medium frequency. High frequency is also used for general radio wave broadcasting and communication, so it is often called as radiofrequency. However there is no exact definition of the word “radiofrequency,” it is generally considered to include a bandwidth from 100kHz to 4MHz. Medtronic® Monopolar SealerTM uses frequencies between 480kHz and 1.6MHz, and Surgitron PellevéTM uses 4MHz in monopolar mode and 1.7MHz in bipolar mode. Thus, these two devices could be called as high frequency or radiofrequency devices.
In incision mode of general electrocautery device, an electric current is passing continuously. As a result, Joule heat is generated from the tip of electrode. This heat makes the tissue fluid boils up rapidly, then the tissue gets dehydrated instantaneously and its value of electric resistance gets increased. According to the increased resistance, the generator also automatically increases its voltage to overcome the resistance, to keep the previously set output (watts). When the peak of the voltage raises more than 200V, an electric break down of a gas, this is also called as arc discharge, is occurred and sparks are generated from the electrode tip. These sparks explode the tissue, and by a longitudinal move of these small tissue explosions, the tissue is “incised” by electrocautery. In this incision mode, generated Joule heat is much by continuous current to incise the tissue, but the peak voltage is not high enough to make much and strong sparks to get hemostasis. In other words, the coagulation is weak in this incision mode.
In coagulation mode of electrocautery, the electric current is passing intermittently not to boil or explode the tissue. Intermittent current does not generate much Joule heat because the total current is small. But the peak voltage is very high, so the sparks are generated much and strong enough to carbonize the tissue without incision. Both incision and coagulation modes could be mixed to get incision and hemostasis at the same time. Each company products usually equipped such blended or mixed mode.
VIO® 300D is a usual general-purpose medium frequency electrocautery using 350kHz. In its low-thermal coagulation mode, which is also called as SOFT COAGTM, the current is continuously passing as a regular incision mode. But the voltage is kept low enough not to explode the tissue. The passing current volume is regulated automatically, usually much increased to raise the tissue temperature. In other words, a large volume of the current is continuously passing at a regulated low voltage. In this condition, much Joule heat is generated without sparks and the tissue is slowly coagulated without incision or carbonization. In both incision and coagulation modes, the tissue temperature is getting to hundreds degrees Celsius (°C), approximately to explode (cut) or carbonize tissue. In soft (low-thermal) coagulation mode, the tissue temperature is regulated between 60 and 100 °C, approximately, not to incise or carbonize but “coagulate” the tissue like a boiled egg.
Generally, the lower the frequency is, and the larger the electric current is, the more harmful the electricity is to a human body. Thus, a radiofrequency device is considered to be safer than a medium frequency device, because the frequency is higher in radiofrequency device than those in general electrocautery device using medium frequency. For example, a large split patient return electrode is needed while SOFT COAGTM mode is used in surgery to avoid serious accidental burn, because it gives a large current to the human body, though VIO® has excellent safe mechanism to avoid such electric injury. But a return electrode should be carefully placed and examined during operation.
Medtronic® Monopolar SealerTM EndoFB 3.0 uses frequencies between 480kHz and 1.6MHz, that are lower than Surgitron PellevéTM that uses 2MHz. Generally, the higher the frequency is, the denser the passing current is on the surface of a conductor. This phenomenon is called as “skin effect.” Therefore, even with the same current volume, the lower the frequency is, the wider and the deeper the heat is infiltrating into the tissue. As Monopolar SealerTM has lower frequency than Surgitron PellevéTM, Monopolar SealerTM shows less dense current than SurgitronTM, so the output should be set at larger power. By Monopolar SealerTM, the heat damage would be wider and the tissue temperature could be higher. To suppress the tissue temperature down to 100°C, Monopolar SealerTM needs simultaneous continuous saline irrigation to the tip during cautery. This saline irrigation does not only cool the tissue temperature but also spread the heat for wider low-temperature coagulation. As Monopolar SealerTM is especial designed for tissue sealing in liver surgery, this could be an advantage for its purpose.
Surgitron PellevéTM is a general-purpose radiofrequency device while Monopolar SealerTM is specialized for tissue sealing. And more, Surgitron PellevéTM uses higher frequency than Monopolar SealerTM, the heat infiltration can be narrower and more superficial, and the tissue temperature stays lower without saline irrigation. The range of coagulation would be more narrowed, and this could be an advantage for coagulation of thin and fragile tissue structures such as pulmonary bullae.
Compared with usual medium frequency electrocautery, Surgitron PellevéTM needs smaller current volume. A patient return electrode could be put even upon the clothes like an “antenna,” because SurgitronTM uses a radio wave. General radiofrequency device shows low conduction speed of smaller energy, and enables to adjust coagulation condition of bullae, not to coagulate too much, easily. Thus, radiofrequency devices are considered to have more advantage in safety and we believe that are more suitable for coagulation of bullae, compared with usual electrocautery.
Results of low-thermal coagulation in combination with or without stapling in dealing with bullae were reported in the literature. Sawabata and coworkers showed good result using spray coagulation by usual electrocautery at low wattage (10-20 watts) in combination with stapling in 2005 [6].
Ambrogi and colleagues reported their experience of pneumothorax treatment by using Monopolar SealerTM alone as an alternative to stapling in 2008, and their long-term results of 73 patients in 2015. The result is excellent, however, they did not show any compared data with stapling bullectomy [1,2]. Fujioka and coworkers also reported the comparison of 251 cases that were treated with Monopolar SealerTM in combination with stapling and 168 case that were treated with stapler alone in the Japanese literature.3 419 cases during 14 years were included in this study. They used Monopolar SealerTM to get thickened the thin bullous tissue alongside the resection line and a surgical stapler was then applied to the thickened pleura. For small bullae, they used only coagulation. They also added fibrin glue and polyglycolic acid sheet in all cases treated with Monopolar SealerTM. They concluded that Monopolar SealerTM reduces the resection volume of the lung and shorten the operative time in comparison of stapler-only group. They did not mention about postoperative persisted air leakage and recurrence rate in this article. Uomoto and coworkers also reported 75 cases that were treated with Monopolar SealerTM as a prospective multiinstitutional study [4]. In this article, for bullae within 3cm in diameter, they use coagulation alone and left them unresected, For the bullae more than 3cm in diameter, they coagulated along the resection line first and get thickened the pleura enough, then resected by using an endoscopic stapler. All treated lesions were covered with polyglycolic acid sheet and fibrin glue. 75 cases including 60 small and 31 large bullae were treated. They also reported that only 3 cases of early postoperative (within 7 days from the operation) air leakage out of the 3 cases of small bullae. Two of them needed surgical treatment again. They also did not report their long-term results.
Pathology demonstrated that radiofrequency cautery successfully make the bulla wall thickened and degenerated without damaging the lung parenchyma deeper than 2mm from the pleural surface. Radiofrequency cautery is a safe, and possibly promising device for low-thermal coagulation of pulmonary bullae in pneumothorax surgery. Especially, general radiofrequency cautery device such as Surgitron PellevéTM is easy to introduce to the pneumothorax surgery in combination with general ball-shaped electrode, because the cost is relatively low (as approximately less than a half as VIO®) and for its safety. Accumulation of the cases is awaited to report its detailed and the long-term results.
We thank Dr. Shin’ichi Nakatsuka for his contribution in pathological diagnoses.
All authors have no conflict of interest.
- Ambrogi MC, Melfi F, Duranti L, Mussi A (2008) Cold coagulation of blebs and bullae in the spontaneous pneumothorax: a new procedure alternative to endostapler resection. Eur J Cardiothorac Surg 34: 911-913. [Crossref]
- Ambrogi MC, Zirafa CC, Davini F, Giarratana S, Lucchi M, Fanucchi O, et al., (2015) Transcollation(R) technique in the thoracoscopic treatment of primary spontaneous pneumothorax. Interact Cardiovasc Thorac Surg 20: 445-448.
- Fujioka S, Uomoto M, Hachisuka Y (2015) Usefulness of Tissue-Link in surgery for primary spontaneous pneumothorax. Nihon Kokyuki Geka Gakkai Zasshi 29: 423-427.
- Uomoto M, Hachisuka Y, Kataoka M, Kawamata O, Hayashi D, Inoue F, et al.,(2008) Usefulness of TissueLink in surgery for lung bullae - The result of a multi-institutional clinical study. Nihon Kokyuki Geka Gakkai Zasshi 22: 135-141.
- Yamamoto S, Otani S, Mitsuda S, Endo T, Hasegawa T, et al., (2011) Electroablation for the treatment of spontaneous pneumothorax using SOFT COAG electrosurgical output system. Kyobu Geka 64: 266-270. [Crossref]
- Sawabata N, Takeda S, Inoue M, Koma M, Tokunaga T, et al., (2005) M-tip electro-ablation of pneumo-cysts for treatment of spontaneous pneumothorax as a secondary method to stapling: a confirmation study. Interact Cardiovasc Thorac Surg 4: 614-617.


