Background: Postmarketing studies have shown a considerable efficacy of natalizumab (NAT) in RRMS, while progressive multifocal leukoencephalopathy (PML) is a well-known risk associated with long-term therapy. To minimize this risk, treatment with NAT is often stopped after 2years. However, the features of clinical and radiological disease activity after NAT withdrawal are still unknown.
Objective: To evaluate effects of NAT discontinuation on clinical and radiological disease activity within twelve months after discontinuation.
Methods: We retrospectively analyzed clinical and radiological data of 30 patients with MS who discontinued NAT between 2006 and 2013.
Results: 14/29 patients had relapses after discontinuation of NAT, 1 patient experienced a rebound phenomenon and 14 patients were relapse-free 12 months after withdrawal. Patients in the relapse group had a higher 1-year pre-NAT treatment ARR (2.44) than the relapse-free group (1.66) (p = 0.0129), while no significant differences were observed comparing EDSS and MRI scores between both groups (p = 0.738 and 0.633 respectively).
Conclusion: Our data suggest that ARR during the year previous NAT treatment start could be a predictor of relapses after NAT withdrawal.
multiple sclerosis, natalizumab, prognosis
Natalizumab (NAT) is a monoclonal antibody directed against the alpha4-integrin which prevents T-cells penetration in the CNS [1] and it is highly effective [2,3]. Due to the risk of PML infection, several patients at high risk discontinue NAT therapy and, despite beginning immunomodulant treatments (IMT), many of them have a disease reactivation [4].
Therefore, considerations on discontinuing an effective treatment for MS as NAT, as well as on the risks of a potentially lethal adverse event such as PML represent a crucial moment in the management of MS [5,6].
The primary aim of this study was to observe the history of the disease after NAT withdrawal, focusing our attention on annualized relapses rate (ARR) and expanded disability status scale (EDSS) scores. Secondly, we tried to identify clinical or radiological markers of disease progression.
Patients
We collected clinical and MR data of 30 patients with clinically definite relapsing-remitting MS (RR-MS) according to the revised McDonald criteria, followed as outpatients at Papa Giovanni XXIII (Bergamo-Italy) and San Gerardo (Monza-Italy) Hospitals, who stopped NAT after at least one year of therapy (39.5 mean infusions ± 17). NAT was started according to the Italian regulatory criteria: (i) patients who did not respond to Interferon Beta (IFN-b) or Glatiramer Acetate (GA) carried out for an adequate period of time (A criterion), or (ii) those having a rapidly worsening disease course, regardless of previous treatment (B criterion) [7].
Reasons for drug discontinuation were anti-JC virus seropositivity combined with long treatment duration and/or previous use of immunosuppressive treatment (n=26) or self-decision based on fear of PML before the availability of anti-JC virus testing (n=4). We excluded patients with high disease activity during NAT treatment, desire for children or pregnancy, persistent antibodies against NAT positivity, allergy or side effects or comorbidities and abroad residency (n=10).
Data collected
The results were retrospectively analyzed; the average follow-up was 18.8 months (ranged from 12 to 24 months); follow-up was calculated from the date of the last infusion.
We retrospectively collected the following clinical parameters: (i) age at NAT start; (ii) disease duration at NAT start; (iii) disease modifying treatments used before NAT therapy; (iiii) relapse frequency and severity; (iiiii) expanded disability status scale
(EDSS) at different milestones: at diagnosis, 12 months before NAT, at NAT start, at withdrawal and 12 months after NAT withdrawal.
Demographic and clinical data of patients are shown in Table 1.
Clinical disease activity recurrence was defined as the appearance or reappearance of one or more symptoms attributable to MS, accompanied by objective deterioration on neurological examination lasting at least 24 h, in absence of fever or and preceded by neurological stability for at least 30 days.
MR was performed after NAT withdrawal and 3-6-12 months later, focusing on the presence of new T2+ lesions and/or Gd-enhancing lesions. Rebound of disease activity was arbitrarily defined as clinical significant increase (at least 2-fold) of ARR in comparison to pre-NAT disease course; one or more severe relapses with sustained disability progression; 5 or more new T2 lesions and/or at least 10 more Gd-enhancing lesions than pre-NAT baseline scan.
For each patient, we calculated the clinical and radiological annualized relapse rates (ARR) in the period from the diagnosis to the year before NAT, during the year previous to NAT treatment, during the administration of therapy and during the following year.
After NAT discontinuation, we proposed to all patients to shift to an alternative therapy: eleven patients (38%) started IMT, either glatiramer acetate (n= 9) or interferon-beta (n= 2) approximately 1.8 month after last NAT infusion (range 1-8 months); the choice between these two first line treatments was based on which treatment the patients did not respond to in the pre-NAT period. Eight additional patients (28%) who discontinued NAT started oral therapy with fingolimod after a mean of 4,1 months (range 3-7); this option has been available since April 2012. The remaining 10 patients (34%) did not start any IMT.
Statistical analysis
Statistical analysis was performed using the Graph-Pad Prism software version 6.0 (GraphPad Inc, La Jolla, CA). Differences in demographic, clinical and radiological characteristics were compared between the patients with relapses during NAT therapy and the patients without relapses during the treatment using independent sample t-test for continuous variables and Fisher exact test for proportion. The same statistical analysis were collected for the group of patients with relapse after NAT therapy and the patients without relapses after NAT therapy.
Student t test for paired data were used to compare the data observed 12 months before NAT, during treatment and 12 months after discontinuing treatment. A p <0.05 was regarded as significant. Univariate correlations were explored using the Pearson correlation coefficient.
Epidemiological and clinical characteristics of the group before-during and after NAT We analyzed 30 patients with RRMS, ten male (33%) and twenty female (67%); mean age (at the suspension) was 39 years + 9 SD (range 16-45) (Table 1).
EDSS score before starting NAT was 3 ± 1.8 SD (range 1-6.5), at the suspension it was 3.15± 2 SD (range 1-6.5), one year after the suspension 3.4± 2.1 SD (range 1-6.5) (Table 1). Mean time of infusion of NAT was 39.5 months ± 17 SD (range 13-74 months) (Table 1). Only 2 patients received NAT as first disease modifying treatment; 22 patients were previously treated with: INF β-1a 1 time/week (3 patients), INF β-1a 3 times/week (10 patients), INF β-1b 3 times/week (7 patients) and with GA (2 patients); 6 patients received at least two disease modifying, and 2 patients were previously treated with mitoxantrone.
Table 1. Demographic and clinical characteristics of study cohort
|
Total |
30 |
F/M |
20/10 |
|
|
Mean |
Standard deviation |
Range
|
Age at the baseline |
|
28.0 |
± 8.6 |
[16-45] |
EDSS at the baseline |
|
1.5 |
± 0.6 |
[0-3] |
Relapse during the 1st year |
|
0.9 |
± 0.9 |
[0-3] |
Relapse during the 1st treatment (GA or IFNB or azathioprine or mitoxantrone) |
|
3.0 |
± 1.5 |
[1-10] |
Treatments before NAT |
|
1.2 |
± 0.7 |
[0-4] |
Number of lesions at the last MR before NAT |
Gd + |
1.8 |
± 0.6 |
[0-6] |
T2 |
1.3 |
± 1.2 |
[0-4] |
ARR in the year before NAT
Disease duration before NAT (months) |
|
1.2
85.7 |
± 1.1
± 85.0 |
[0-4]
[4-307] |
EDSS pre-NAT |
|
3.0 |
± 1.8 |
[1-6,5] |
Relapses in NAT |
|
0.5 |
± 0.7 |
[0-2] |
Age at the suspension |
|
39 |
± 9 |
[21-56] |
Number of infusions of NAT |
|
39.5 |
± 17 |
[13-74] |
EDSS at the suspension |
|
3.15 |
± 2 |
[1-6,5] |
EDSS 1 year later the suspension |
|
3.4 |
± 2.1 |
[1-6,5] |
Eleven patients out of 30 (36.7%) have had at least one relapse during NAT treatment (Table 2). Five of these eleven patients (45%) have had relapses also after NAT withdrawal.
Patients with relapse during NAT treatment vs patients’ disease free
The mean time between the diagnosis and the beginning of NAT was 118.63 months ± 102. 01 SD months in the group with at least one relapse during NAT treatment; in the other group was 66.73 months ± 58.30 SD (p = 0.0380) (Table 2; Figure 1).
EDSS score at the beginning of the treatment was 2.9± 1.7SD (range 1-6.5) in the first group; in the second one, the EDSS score was 3.2± 1.9DS (1-6.5) (p = 0.773) (Table 2).
EDSS score at the withdrawal of NAT was 2.9± 2.1SD (range 1-6.5) in the first group, and 3.5± 2.0SD (range 1-6.5) in the second one (p = 0.497) (Table 2).
Mean EDSS was not significantly different between the two groups at the starting (p = 0.78), at the withdrawal (p = 0.67) and one-year after NAT treatment (p = 0.94) (Figure 1).
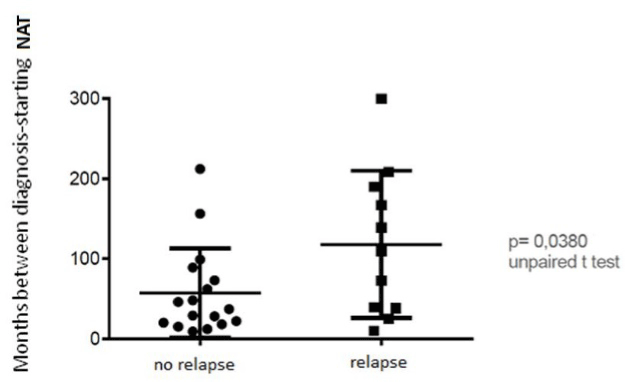
Figure 1. Months between diagnosis and starting NAT: there is a significant different in the mean time from the diagnosis to the beginning of NAT between the group with at least one relapse during NAT treatment (118.63 months ± 102. 01 SD months) and the other group (66.73 months ± 58.30 SD months) (p = 0.0380).
ARR score in the year before starting NAT was 1.3±1.1SD (range 0-4) in the group of patients with relapse during NAT and 1.2±1.1SD (range 1-3) in the other group (Table 2).
There was not a significant different in the ARR score between two groups (p = 0.851) (Table 2).
Table 2. Patients characteristics after NAT withdrawal
|
|
Patients with relapse after NAT withdrawal |
Patients with no relapse after NAT withdrawal |
P value |
Total |
15 |
15 |
|
F/M |
10/5 |
10/5 |
|
|
|
Mean |
Standard deviation |
Range
|
Mean |
Standard deviation |
Range
|
|
Age at the baseline |
|
27.6 |
± 7.8 |
[17-39] |
28.4 |
±9.5 |
[16-45] |
0.869
|
EDSS at the baseline |
|
1.4 |
± 0.6 |
[0-2.5] |
1.6 |
± 0.6 |
[1-3] |
0.4137
|
Relapse during the 1st year |
|
0.9 |
± 0.9 |
[0-3] |
1.0 |
± 0.9 |
[0-3] |
0.7301
|
Relapse during the 1st treatment (GA or IFNB or azathioprine or mitoxantrone) |
|
2.8 |
± 2 |
[1-5] |
3.9 |
± 1.7 |
[2-10] |
0.6244
|
Treatments before NAT |
|
1.4 |
± 0.8 |
[1-4] |
1.1 |
± 1.1 |
[0-3] |
0.4887
|
Number of lesions at the last MR before NAT |
Gd + |
2.1 |
± 1.5 |
[0-6] |
1.7 |
± 1.1 |
[0-5] |
0.3934
|
T2 |
0.8 |
± 1.3 |
[0-4] |
0.5 |
± 1 |
[0-4] |
0.7215
|
ARR before NAT |
|
2.44 |
± 0.9 |
[1-4] |
1.66 |
± 0.7 |
[1-3] |
0.0129 |
EDSS pre-NAT |
|
3.1 |
± 1.9 |
[1-6.5] |
3.4 |
± 1.7 |
[1-6.5] |
> 0.9999 |
Relapses in NAT |
|
0.4 |
± 0.6 |
[0-2] |
0.8 |
± 0.9 |
[0-2] |
0.2496 |
Age at the suspension |
|
40.2 |
± 7.8 |
[25-56] |
37.9 |
± 10 |
[21-54] |
0.4516 |
Number of infusions of NAT |
|
38.3 |
± 15 |
[13-74] |
45.1 |
± 18 |
[13-70] |
0.4177 |
EDSS at the suspension |
|
3.2 |
± 2.1 |
[1-6.5] |
3.5 |
± 2.1 |
[1- 6.5] |
0.9663 |
EDSS 1 year later the suspension |
|
3.5 |
± 2.1 |
[1-6.5] |
3.3 |
± 2.2 |
[1- 6.5] |
0.9562 |
Relapse rate and disability before and after treatment
Mean relapse rate decreased significantly in the first year of treatment and remained stable for all time of the treatment. Mean ARR decreased from 1.2 ± 1.1 SD in the year prior the treatment to 0.6 ± 0.5 SD (p <0.0001) during NAT (Figure 2).
Even one-year after NAT withdrawal the mean ARR resulted significantly lower (0.88 ± 0.71 SD) than the year before the treatment (p < 0.0001) (Figure2).
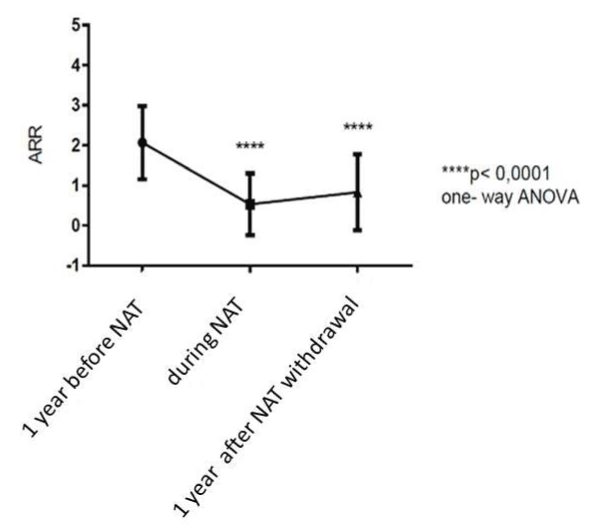
Figure 2. Rappresentation of annualized relapse rate (ARR) before, during and after NAT-treatment: mean relapse rate decreased significantly in the first year of treatment with NAT and remained stable for all time of the treatment. ARR resulted significantly lower even one-year after NAT withdrawal.
Patients with EDSS score ≤3 before treatment, at the withdrawal and one-year later were 14/30 (47%). Patients with EDSS score = 6.5 before starting NAT were 3/30 (10%); when NAT was stopped were 6/30 (20%); one year later the patients were 7/30 (23%). Before starting NAT, disease duration was similar in patients with EDSS score ≤ 3 and the group of patients with EDSS score>3 (p = 0.15). No correlation was found between relapse rate (ARR) during NAT and disability gain (EDSS) (Rs=0.065).
Time of first relapse after NAT withdrawal
After withdrawal of NAT a remarkable increase of disease activity occurred.
The mean time of the first relapse was 26 weeks. The earliest relapse occurred after 11 weeks, the latest after 45 weeks.
Overall, 13% (4/30) had only one relapse in the year after withdrawal; 30% (9/30) had two relapses and 7% (3/30) had at least 3 relapses. Only one patient (3%) had a rebound phenomenon. 17% (5/30) had a relapse during NAT and after withdrawal of the treatment.
Radiological relapses (defined as the presence of one or more contrast-enhancing lesions on post-contrast T1-weighted images and/or development of one or more new hyper intense lesions on T2-weighted sequences (compared with previous scan)) occurred earlier than the clinical one.
Patients with relapse after NAT withdrawal vs patients’ disease free
There was no difference in sex, age, disease duration and number of infusions of NAT between the group of patients with relapse after NAT withdrawal and the disease-free patients. The two groups differed for ARR in the year previous NAT (p = 0.0129) (Figure 3).
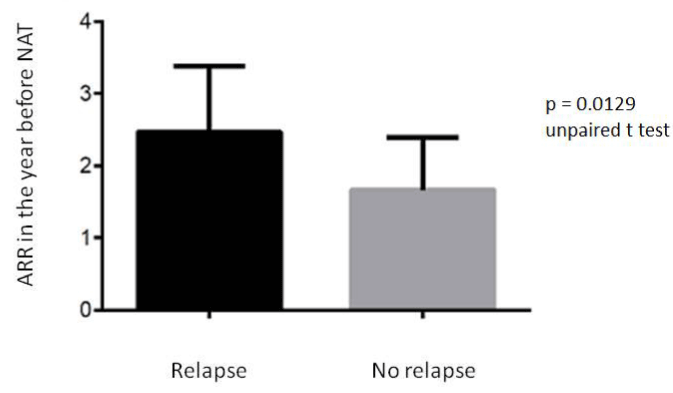
Figure 3. ARR in the year previous NAT in the group of patients with relapse after NAT withdrawal and in the group disease free. The two groups differed for (p = 0.0129).
Anyway, EDSS score was similar between the two groups before starting NAT (p = 0.41), at the suspension (p = 0.74) and one year after the withdrawal (p = 0.72).
Moreover, we stratified the relapses by timing of presentation: 4 patients out of 15 (27%) relapsed within 3 months after NAT withdrawal.
These patients had a disease duration longer than the patients who relapsed after the third month; respectively 182.25 months ± 114. 40 SD vs. 75. 4 months ± 89.01SD (p = 0.045). Furthermore, the patients with early relapses had an EDSS score before treatment higher than the others (p = 0.008) (Figure 4); also at the suspension of NAT, the EDSS score was higher in this group (p = 0.011) (Figure 5).
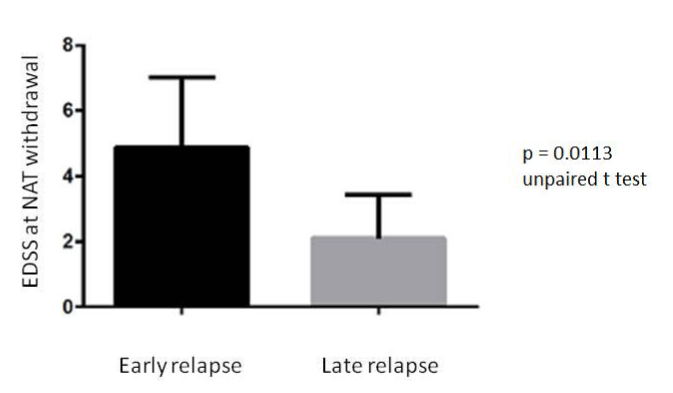
Figure 4. Difference in EDSS score at NAT withdrawal in the group of patients with early relapse and in the group with late relapse after NAT suspenction.

Figure 5. Difference in ARR score before NAT treatment in the group of patients with early relapse and in the group with late relapse after NAT withdrawal.
ARR before NAT was not significantly different between both groups (p = 0. 335) (Figure 6).
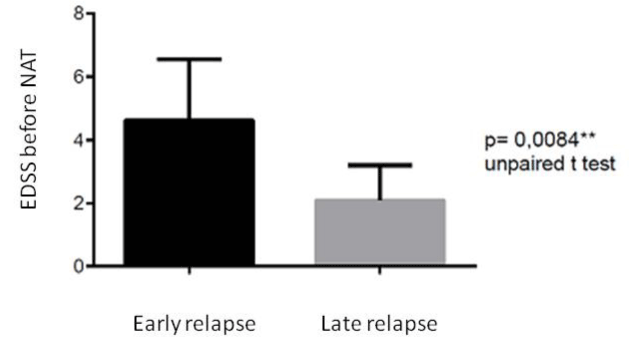
Figure 6. Difference in EDSS score before NAT treatment in the group of patients with early relapse and in the group with late relapse after NAT withdrawal.
NAT is extremely effective in reducing disease activity in RRMS [8], but the long-term treatment with this drug has been associated with an increased risk of PML [9].
The overall prevalence of PML among NAT-treated MS patients has been estimated at 1 case/1000 patients in studies dating back from the first years the drug was introduced [10].
However, additional cases have occurred since then, and 514 PML cases have been reported in 129.100 patients who received at least 1 dose of NAT up to December 2014 [11].
Our observations show that a long-time lapse between diagnosis and starting of NAT therapy determines a higher risk of relapses during treatment. On the contrary, an early initiation of NAT therapy seems to grant a longer relapse-free period; these observations are in line with those of Putzki et al. [12]. Importantly, NAT has been available in Italy only since 2006 and was initially sought as a treatment option for patients with long disease duration; hence, the bias of a wide therapeutic window has not always been conditioned by strict clinical motivations.
The return of disease activity following NAT treatment interruption most likely reflects a resumption of lymphocyte migration across endothelial membranes as NAT is cleared from circulation [13]. Steady-state concentrations of NAT is achieved in the serum after approximately 6 months of repeated monthly infusions, and the elimination half-life is approximately 11 days (TYSABRI® (NAT) package insert. Cambridge, MA: Biogen Idec Inc.; 2008). Based on this pharmacokinetic profile, NAT should be fully cleared from circulation approximately 2 months (5 half-lives) after the last dose13. According to this observation, we found that the mean time of the first relapse after NAT withdrawal was above 26 weeks, in line with the observations of Hoepner et al. [14].
Many studies agreed in observing a more prominent disease activity return after NAT withdrawal [15-18], even though the markers of baseline disease activity have not been explicited. Only Prosperini et al. [19] have recently found that the risk of disability worsening, expecially in patient with EDSS over 3.0. Even if it is important to identify a biomarker of an aggressive disease for a tailored treatment, few authors analyzed patients’ history before starting NAT and to date none of them considered radiological aspects.
Our data, as reported by O’Connor et al [20], revealed that a high ARR pre-NAT could be a predictor of disease activity in NAT treated-patients. In our study the RRMS activity reaches baseline levels in a time frame consistent with NAT elimination kinetics and is apparently unaffected by the duration of the disease activity. Moreover, the disease activity return was particularly noticeable in patients with highly active disease before NAT treatment. These data suggest that patients with high ARR pre-NAT could need an aggressive treatment after NAT and a quick switch to another therapy.
Accordingly, ARR pre-NAT together with the risk stratification through detection of anti-JCV antibody status [21-22], the EDSS score at NAT start [23] should be considered before starting any treatment. In fact, these parameters seem to be closely associated to a higher risk of relapses at NAT withdrawal and to a worsening of EDSS.
It is questioned if rebound phenomenon represents a real clinical entity: most reports of rebound after discontinuation of NAT arise from rather small studies in selected patients and single-center cohort observations (Table 3) [16,17,24,25,28,30], and some authors like O’Connor et al. [20] didn’t show evidence of rebound. Also in the perspective study
Table 3. Patients characteristics during NAT treatment
|
|
Total |
Relapse during NAT |
Disease free during NAT |
P value |
Total |
30 |
11 |
19 |
|
F/M |
20/10 |
6/5 |
14/5 |
|
|
Mean |
SD |
Range |
Mean |
SD |
Range |
Mean |
SD |
Range
|
|
Number of lesions at the last MR before NAT
|
Gd + |
1.8 |
± 0.6 |
[0-6] |
1.3 |
± 0.7 |
[0-6] |
2.0 |
± 1.5 |
[0-5] |
0.101 |
T2 |
1.3 |
± 1.2 |
[0-4] |
0.6 |
± 1.4 |
[0-4] |
0.6 |
± 1.2 |
[0-4] |
0.942 |
Desease duration NAT (months) |
|
85.7 |
± 85.0 |
[4-307] |
118.63 |
± 102. 01 |
[12-307] |
66.73 |
± 58.3 |
[4-190] |
0.0380 |
ARR pre-NAT |
|
1.2 |
± 1.1 |
[0-4] |
1.3 |
± 1.1 |
[0-4] |
1.2 |
± 1.1 |
[1-3] |
0.851 |
EDSS pre-NAT |
|
3.0 |
± 1.8 |
[1-6.5] |
2.9 |
± 1.7 |
[1-6.5] |
3.2 |
± 1.9 |
[1-6.5] |
0.773 |
Relapse during NAT |
|
0.5 |
± 0.7 |
[0-2] |
1.5 |
± 0.5 |
[0-2] |
0 |
± 0.0 |
[0-0] |
0.00001 |
EDSS at the suspension |
|
3.15 |
± 2 |
[1-6.5] |
2.9 |
± 2.1 |
[1-6.5] |
3.5 |
± 2.0 |
[1- 6.5] |
0.497 |
EDSS after 1 year |
|
3.4 |
± 2.1 |
[1-6.5] |
3.4 |
± 2.0 |
[1-6.5] |
3.1 |
± 1.1 |
[1- 6.5] |
0.718 |
TY-STOP on 124 patients who discontinued NAT after 24 doses, the phenomenon of rebound MS was not observed [27].
However, many different definitions of rebound have been proposed: the development of new and/or enlarging T2 lesions [28], relapse severity and number of gadolinium-enhancing lesions16, relapse severity with sustained EDSS worsening [29], or ARR and gadolinium-enhancing lesions and worsening of disease activity beyond pretreatment levels as measured by ARR and EDSS [30]. We arbitrarily adopted a definition of rebound as a clinical significant increase (at least 2-fold) of ARR in comparison to pre-NAT disease course; one or more severe relapses with sustained disability progression; 5 or more new large T2 lesions and/or at least 10 more Gd-enhancing lesions than pre-NAT baseline scan, this definition was felt as the most exhaustive. (Table 4)
Table 4. Summary of the main article on literature
|
[30] |
[28] |
[25] |
[24] |
[17] |
[16] |
Present study |
Number of subjects who stopped NAT |
137 |
21 |
68 |
10 |
1517 |
27 |
30 |
Mean number of relapses in the year
before NAT start |
1.5 |
1.2 |
1.9 |
1.8 |
Not avaible |
2.3 |
1.3 |
NAT treatment duration
(months) |
6 |
From 1 to 37 |
>12 |
>12 |
From 1 to 41 |
From 6 to 23 |
>24
(2 patients >13) |
Follow up duration after stopping NAT
(months) |
12 |
15 |
6 |
6 |
8 |
6 |
>12 |
Number of patients with relapse during
follow up |
44/137 (32%) |
Not avaible |
19/68 (28%) |
7/10 (70%) |
21% |
18/27 (67%) |
15/30 (50%) |
Number of patients treated with another drug
during follow up |
Not avaible |
Not avaible |
4/68 (4%) |
0/10 (0%) |
402/946 (42%) |
0/27 (0%) |
20/30 (66%) |
Observation of severe relapses and/or unusual
inflammatory activity on MR |
Observed |
7/19
|
7/10 |
No |
4/27 |
1/30 |
Adopting this definition, only one patient presented a rebound activity in our study. We cannot offer an explanation for this finding since this patient did not present distinctive features from the others.
Our data analysis clearly demonstrates that the flare-up of disease activity was primarily seen in MR and less in relapse activity. Also, Borriello et al. [31] reported this result, but it wasn’t replicated in the RESTORE [32] study, a randomized, partially placebo-controlled exploratory study evaluating MS disease activity during 24-week interruption of NAT. Though, the same authors concluded that the earlier recurrence of clinical rather than radiologic disease may also reflect the more subjective nature of clinical relapse reporting, independently from EDSS changing.
In order to prevent relapses following NAT withdrawal, patients are usually switched to another treatment. Considering that 50% of our cohort showed signs of disease reactivation after discontinuation, patients should be closely monitored to detect clinical and/or radiological disease activity as soon as possible in order to ready modulate preventive therapy or even to return to NAT therapy despite PML risk.
Moreover, a preventive treatment should be started even there is only a radiological reactivation in order to limit the stepwise accumulation of disability.
Based on the data of the present study we cannot suggest a preventive treatment which is at the same time safe and effective after NAT discontinuation: as a matter of fact, it is clear that interferon- beta [33] or glatiramer acetate [34] are not effective enough to prevent relapses, whereas fingolimod seems to increase the risk of PML [4,35].
So, in patients with high ARR in the year before starting NAT and patients with EDSS>3 probably the maintenance of NAT would be a chance for these patients.
We think that the main result emerging from this observational study on a small cohort is that NAT should be used as soon as possible to achieve its main efficacy and lower risks.
All authors have nothing to declare.
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, et al. (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol 58: 840-846. [Crossref]
- Havrdova E, Galetta S, Hutchinson M, Stefoski D, Bates D, et al. (2009) Effect of natalizumab on clinical and radiological disease activity in ultiplesclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 8: 254-260. [Crossref]
- Melin A, Outteryck O, Collongues N, Zéphir H, Fleury MC, et al. (2012) Effect of natalizumab on clinical and radiological disease activity in a French cohort of patients with relapsing-remitting multiple sclerosis. J Neurol 259: 1215-1221. [Crossref]
- Gajofatto A, Turatti M, Monaco S, Benedetti MD (2015) Clinical efficacy, safety, and tolerability of fingolimod for the treatment of relapsing-remitting multiple sclerosis. Drug Healthc Patient Saf 7: 157-1567. [crokssref]
- Fernández O1 (2013) Best practice in the use of natalizumab in multiple sclerosis. Ther Adv Neurol Disord 6: 69-79. [Crossref]
- Havla J, Kleiter I, Kümpfel T (2013) Bridging, switching or drug holidays - how to treat a patient who stops natalizumab? Ther Clin Risk Manag 9: 361-369. [Crossref]
- Mancardi GL, Tedeschi G, Amato MP, D'Alessandro R, Drago F, et al. (2011) Three years of experience: the Italian registry and safety data update. Neurol Sci 31 Suppl 3: 295-297. [Crossref]
- Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354: 899-910. [Crossref]
- Gorelik L1, Lerner M, Bixler S, Crossman M, Schlain B, et al. (2010) Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 68: 295-303. [Crossref]
- Yousry TA1, Major EO, Ryschkewitsch C, Fahle G, Fischer S, et al. (2006) Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 354: 924-933. [Crossref]
- Biogen Idec data, December 2014.
- Putzki N, Yaldizli O, Mäurer M, Cursiefen S, Kuckert S, et al. (2010) Efficacy of natalizumab in second line therapy of relapsing-remitting multiple sclerosis: results from a multi-center study in German speaking countries. Eur J Neurol 17: 31-37. [Crossref]
- Niino M, Bodner C, Simard ML, Alatab S, Gano D, et al. (2006) Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol 59: 748-754. [Crossref]
- Hoepner R, Havla J, Eienbröker C, Tackenberg B, Hellwig K, et al. (2014) Predictors for multiple sclerosis relapses after switching from natalizumab to fingolimod. Mult Scler 20: 1714-1720. [Crossref]
- Havla J, Gerdes LA, Meinl I, Krumbholz M, Faber H, et al. (2011) De-escalation from natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 258: 1665-1669. [Crossref]
- Kerbrat A, Le Page E, Leray E, Anani T, Coustans M, et al. (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci 308: 98-102. [Crossref]
- O'Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, et al. (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76: 1858-1865. [Crossref]
- Sorensen PS, Koch-Henriksen N, Petersen T, Ravnborg M, Oturai A, et al. (2014) Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 261: 1170-1177. [Crossref]
- Prosperini L, Annovazzi P, Capobianco M, Capra R, Buttari F, et al. (2015) Natalizumab discontinuation in patients with multiple sclerosis: Profiling risk and benefits at therapeutic crossroads. Mult Scler 21: 1713-1722. [Crossref]
- O'Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, et al. (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76: 1858-1865. [Crossref]
- Plavina T, Berman M, Njenga M, Crossman M, Lerner M, et al. (2012) Multi-site analytical validation of an assay to detect anti-JCV antibodies in human serum and plasma. J Clin Virol 53: 65-71. [Crossref]
- Sørensen PS, Bertolotto A, Edan G, Giovannoni G, Gold R, et al. (2012) Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 18: 143-152. [Crossref]
- Stüve O, Marra CM, Jerome KR, Cook L, Cravens PD, et al. (2006) Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 59: 743-747. [Crossref]
- Killestein J, Vennegoor A, Strijbis EM, Seewann A, van Oosten BW, et al. (2010) Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 68: 392-395. [Crossref]
- West TW, Cree BA (2010) Natalizumab dosage suspension: are we helping or hurting? Ann Neurol 68: 395-399. [Crossref]
- Lenhard T, Biller A, Mueller W, Schönberger J, and Wildemann B (2010) Immune reconstitution inflammatory syndrome after withdrawal of natalizumab? Neurology 75: 831-833. [Crossref]
- Clerico M, Schiavetti I, De Mercanti SF, Piazza F, Gned D et al. (2014) Treatment of relapsing-remitting multiple sclerosis after 24 doses of natalizumab: evidence from an Italian spontaneous, prospective, and observational study (the TY-STOP Study). JAMA Neurol 71: 954-960. [Crossref]
- Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH (2008) Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology 70: 1150-1151. [Crossref]
- Vidal-Jordana A, Tintoré M, Tur C, Pérez-Miralles F1 Auger C, et al. (2015) Significant clinical worsening after natalizumab withdrawal:Predictive factors. Mult Scler 21: 780-785. [Crossref]
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, et al. (2003) International Natalizumab Multiple Sclerosis Trial Group. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 348: 15-23. [Crossref]
- Borriello G, Prosperini L, Marinelli F, Fubelli F, Pozzilli C (2011) Observations during an elective interruption of natalizumab treatment: a post-marketing study. Mult Scler 17: 372-375. [Crossref]
- Kaufman M, Cree BA, De Sèze J, Fox RJ, Gold R, et al. (2015) Radiologic MS disease activity during natalizumab treatment interruption: findings from RESTORE. J Neurol 262:326-336. [Crossref]
- Gobbi C, Meier DS, Cotton F, Sintzel M, Leppert D, et al. (2013) Interferon beta 1b following natalizumab discontinuation: one year, randomized,prospective, pilot trial. BMC Neurol 13: 101. [Crossref]
- Rossi S, Motta C, Studer V, De Chiara V, Barbieri F, et al. (2013) Effect of glatiramer acetate on disease reactivation in MS patients discontinuing natalizumab. Eur J Neurol 20: 87-94. [Crossref]
- Maillart E, Louapre C, Lubetzki C, and Papeix C (2014) Fingolimod to treat severe multiple sclerosis after natalizumab-associated progressive multifocal leukoencephalopathy: a valid option? Mult Scler 20: 505-509. [Crossref]






