A pooled analysis [1] does not found worse safety profiles in patients reaching very low values of LDL-Cholesterol (<25 and <15 mg/dl) compared to higher LDL-Cholesterol levels. Authors concluded that aggressive alirocumab therapy seems generally well tolerated. We raise some methodological questions.
First, Authors’ analyses used propensity scores from regression models lacking in predictors of safety endpoints, while covariates must be associated to both outcome and exposition: associations with exposition only affect the analytical precision and do not adjust for possible confounders [2].
Second, limiting the evaluation to alirocumab therapies and focusing to very-low LDL-Cholesterol values subgroups reduces the power of comparisons and increases the risk of false negatives. The apparent lack of association between the LDL-Cholesterol <25 and <15 mg/dl and the risk of neurocognitive disorders can be due to the scarce power of these analyses. Indeed, the sample size of the groups exposed to those targets [1] can demonstrate with sufficient statistical power (≥80%), only very large hypothetical increases in risk of neurocognitive events (RRs ≥2.6 and ≥3.4 respectively).
PCSK9-abs are impressively effective in LDL-Cholesterol lowering, but their effectiveness on clinical major endpoints is uncertain. Our critical revision [3] of two PCSK9-abs meta-analyses [4,5] arouses more caution. After corrections, Navarese’s results [4] lost statistical significance and Lipinski’s results were resized [Death OR=0.51 (0.30-0.85); Neurocognitive Events OR=1.79 (1.05-3.06)].
Metaregression analyses [3] found no association between LDL-Cholesterol lowering and all-cause mortality [for every standard error of LDL-Cholesterol lowering: ratio of OR=0.99 (p=0.503)] and a significant log-linear association with neurocognitive events [for every standard error of LDL-Cholesterol lowering: ratio of OR=1.03 (p=0.042)]. (Figure 1)
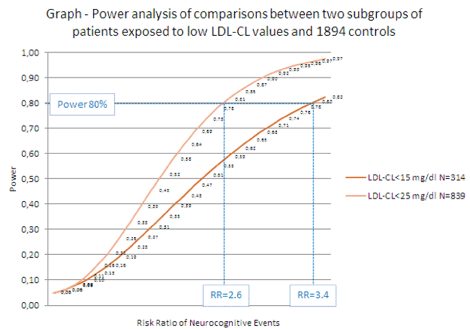
Figure 1. Graph-Power analysis of comparisons between two sub groups of patients exposed to low LDL-CL values and 1894 controls.
- Robinson JG, Rosenson RS, Farnier M, Chaudhari U, Sasiela WJ, et al. (2017) Safety of Very Low Low-Density Lipoprotein Cholesterol Levels With Alirocumab: Pooled Data From Randomized Trials. J Am Coll Cardiol 69: 471-482. [Crossref]
- Garrido MM1, Kelley AS, Paris J, Roza K, Meier DE, et al. (2014) Methods for constructing and assessing propensity scores. Health Serv Res 49: 1701-1720. [Crossref]
- Battaggia A, Scalisi A, Schivalocchi A, Donzelli A (2016) Communication “Revisione delle evidenza di letteratura sul trattamento farmacologico del colesterolo”. XXV Seminario Nazionale ISS.
- Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, et al. (2015) Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults with Hypercholesterolemia: A Systematic Review and Meta-analysis. Ann Intern Med 163: 40-51. [Crossref]
- Lipinski MJ, Benedetto U, Escarcega RO (2016) The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 37: 536-545.
2021 Copyright OAT. All rights reserv
Editorial Information
Editor-in-Chief
Massimo Fioranelli
Guglielmo Marconi University
Article Type
Letter to Editor
Publication history
Received date: March 04, 2017
Accepted date: March 15, 2017
Published date: March 17, 2017
Copyright
© 2017 Alessandro Battaggia. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Battaggia A, Scalisi A, Schivalocchi A, and Donzelli A (2016) PCSK9-AB and risk of neurocognitive events – comments to Robinson’s meta-analysis. J Integr Cardiol 3: DOI: 10.15761/JIC.1000211
Corresponding author
Alessandro Battaggia
Foundation Allineare Sanità e Salute – c/o Studio Tracanella Via C.G. Merlo, 3 - 20122 Milano Italy

