Introduction: Most cutaneous head and neck tumors have a proclivity to spread via the lymphatic system and those sited in the upper face and scalp commonly drain to parotid lymph nodes. Sentinel Node Biopsy (SNB) allows the clinician to reliably harvest and then evaluate the first node to receive lymph drainage. Classically superficial parotidectomy would be required to retrieve the SN lodged in the parotid so limiting its utility. The current study utilizes a minimally invasive technique of parotid surgery that makes harvesting of the parotid SN a safe and reliable technique. Sentinel nodes procedure and facial nerve monitoring in order to protect the facial nerve.
Materiel and methods: A retrospective study was performed and twenty two patients suffering from cutaneous head and neck tumors requiring a sentinel node biopsy benefited from the combination of this procedure. Facial nerf identification and injury were registered.
Results: In all cases nerve monitoring enable to identify and monitoring the facial nerve and the sentinel node(s) were identified. These sentinel nodes were negative for 16 patients, positive for six patients. After surgery, no complication was observed.
Conclusion: This current experience confirms that the intra-parotid sentinel node biopsy combined with the nerve monitoring may be a safe and mini-invasive technique, which reduces the risk of facial nerve injuries as well as the operative time.
head and neck tumor, sentinel lymph node biopsy, nerve monitoring, parotid
Whatever their histology (melanoma,meibomian gland carcinoma, Merkel cell carcinoma & squamous cell cancer) , most of the cutaneous head and neck tumors have a proclivity to spread via the lymphatic system with a risk of nodal metastasis [1]. Although the lymphatic drainage is not predictable, many cutaneous tumors of the upper face and scalp have a tendancy to drain to the parotid lymph nodes (70.6%) [2,3]. In patient’s staged as No, sentinel node biopsy (SNB) allows the clinician to reliably harvest and then evaluate the first node to receive lymph drainage [4]. No longer does the oncologist have to estimate the risk of early metastasis and gamble on the appropriate treatment pathway. It is now possible to eliminate chance and determined definatively by SNB whether an early metastasis has occured. This introduces for the first time personalised care to head and neck cancer patient.
Harvesting sentinel nodes in the parotid would normally require a traditional superficial parotidectomy with facial nerve dissection. The time entailed and mordbidity associated with this surgery has detracted from using SNB in this context. But a minimally invasive approach to parotid surgery has been described that reduces these constraints [5]. The risk to the facial nerve is further reduced by the adoption of intraoperative nerve monitoring (IONM). The aim of this study was to assess if SN’s in the parotid gland could be harvested safely by SNB minimally invasive parotid surgery.
Between october 2007 and june 2016, a retrospective review of all the patients who benefited from SNLB by way of minimally invasive parotid surgery in conjunction IOIM was undertaken. This involved a range of histological tumour types (Table 1). The common feature of this study group was the presence of the SN in the parotid gland and the need to retrieve it to asses metastatic spread of cancer.
Surgical technique
All the patients received an injection of a radioactive-tracer (99m Technetium colloidal rhenium sulfide [99mTc NANOCIS CIS bio international, Gif sur Yvette, France]) with lymphoscintigraphy the day before the surgery. A dose of 2 or 4 x 15 mBq (Becquerel, or 2 x 0.4mCi) in 0.2 mL volume was injected in the peri-tumoral area. Tomoscintigraphy was performed 1 hour later with a double head gamma-camera associated to CT scan in order to facilitate the location (SPECT-CT) (Figure 1).
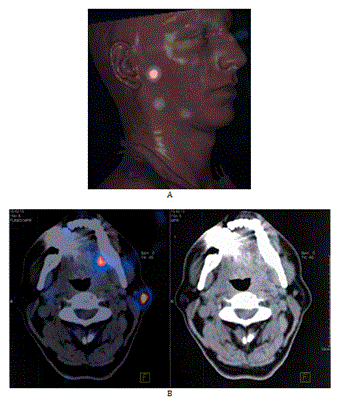
Figure 1. Exemple of preoperative imaging of SPECT-CT highlighting the nodes mapping. A) exemple of 3D recontruction. B) exemple of SPECT-CT slides.
Surgery was performed under general anesthesia without local anesthesia. The surgeon used a combination of pre-operative SPECT-CT to identify the general anatomical location of the node and at surgery a hand held gamma probe was used to be guided the operator onto the SN (Europrobe II, small probe semiconductor Cadmium, EM instrument, France). The technique of extra capsular dissection [5] was used to harvest the SN. A limited pre or infra-auricular cutaneous incision was used to expose the parotid fascia and allowing incision through the fascia directly above the SN. The technique was adapted to the requirements of each patient. The SN was said superficial when it was located under the capsula and deeply situated when it was intra glandular either intra superficial lobe or in the parotid tail. Deeply SN was relatively easy to harvest by a secure approach between the upper and lower branche of the facial nerve of the facial nerve through the nerve detection Once the node was identified it was removed carefully without violating its capsule (Figure 2). The use of IONM helped minimize any risk to the facial nerve (Figure 3). Once hemostasis was complete the incision in the parotid capsule was reapprocimated and the skin closed with non-absorbable sutures (Ethylene 5/0).
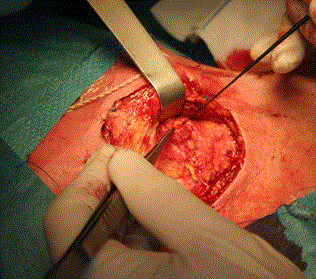
Figure 2. Exemple of the needle electrodes positoning with the NIM (NIM-3; Medtronic Xomed, Jacksonville, FL).
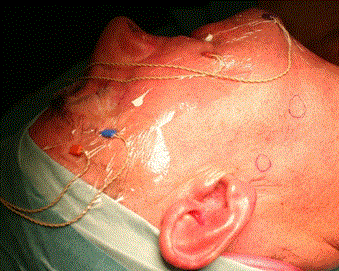
Figure 3. A limited incision of superficial parotid capsula allowed to access to the nodes which was intraoperatively spotted by a handheld gamma camera
Twenty two patients had parotid surgery to retrieve SN between October 2007 to june 2016. The mean age of patients was 66 y (range 28-86y). A SN sentinel lymph was identified and retrieved in all cases. A mean of 4,3 nodes per patient (range 1-8) was found, 29 nodes were in the superficial part of the parotid and 21 nodes were lay deep to the facial nerve trunk.
The SNLB was negative 16 patients and positive for metastatic disease in 6 (including one case of ethmoid adenocarcinoma isolated cells and one case of micrometastasis). The results are summarized in table 1.
Table 1. Summary of the patient’s data.
|
Patient
|
Age
|
Sex
|
Localization
|
Histology
|
Nodes
|
|
Superficial
|
Deep
|
Total
|
SN positif
|
|
1
|
68
|
M
|
right eye
|
melanoma
|
2
|
0
|
2
|
0
|
|
2
|
61
|
M
|
right eye
|
melanoma
|
1
|
1
|
2
|
0
|
|
3
|
58
|
F
|
scalp
|
melanoma
|
3
|
0
|
3
|
1
|
|
4
|
77
|
M
|
right upper eyelid
|
squamous cell carcinoma
|
1
|
1
|
2
|
0
|
|
5
|
76
|
M
|
retro-auricular
|
squamous cell carcinoma
|
2
|
1
|
3
|
0
|
|
6
|
61
|
M
|
right eye
|
squamous cell carcinoma
|
0
|
1
|
1
|
0
|
|
7
|
66
|
F
|
right upper eyelid
|
merckel carcinoma
|
2
|
2
|
4
|
0
|
|
8
|
77
|
M
|
right lower eyelid
|
annexiel carcinoma
|
2
|
0
|
2
|
0
|
|
9
|
54
|
M
|
right lower eyelid
|
sebaceous carcinoma
|
1
|
0
|
1
|
0
|
|
10
|
51
|
M
|
ethmoid sinus relapsing in the orbit
|
adenocarcinoma
|
0
|
1
|
1
|
1
|
|
11
|
52
|
M
|
right eye
|
squamous cell carcinoma
|
2
|
0
|
2
|
0
|
|
12
|
60
|
M
|
right cheek
|
melanoma
|
2
|
0
|
2
|
1
|
|
13
|
66
|
M
|
cervical left
|
melanoma
|
1
|
1
|
2
|
0
|
|
14
|
83
|
M
|
right eye
|
squamous cell carcinoma
|
5
|
3
|
8
|
1
|
|
15
|
83
|
M
|
left temporal
|
melanoma
|
0
|
1
|
1
|
1
|
|
16
|
79
|
F
|
left lupper eyelid (recurrence)
|
squamous cell carcinoma
|
0
|
2
|
2
|
0
|
|
17
|
28
|
M
|
right lower eyelid
|
squamous cell carcinoma
|
0
|
1
|
1
|
0
|
|
18
|
56
|
M
|
left lower eyelid
|
sebaceous carcinoma
|
2
|
1
|
3
|
1
|
|
19
|
74
|
M
|
left lower eyelid
|
sebaceous carcinoma
|
1
|
0
|
1
|
0
|
|
20
|
80
|
F
|
right cheek
|
squamous cell carcinoma
|
1
|
0
|
1
|
0
|
|
21
|
86
|
M
|
left ear
|
squamous cell carcinoma
|
1
|
4
|
5
|
0
|
|
22
|
57
|
M
|
left eye
|
squamous cell carcinoma
|
0
|
1
|
5
|
0
|
|
Total
|
|
|
|
|
29
|
21
|
50
|
6
|
|
Mean
|
66,05
|
|
|
|
1,44
|
0,95
|
4,35
|
0,27
|
There were no incidents of facial nerve injury or other complications in this small series of cases.
2021 Copyright OAT. All rights reserv
The majority of cutaneous cancers in the head and neck have an affinity for lymphatic dissemination. The drawback is that the lymphatic drainage in the head and neck from any one anatomical area is not predictable [6]. This give SLNB it advantage [7].
Sentinel lymph node biopsy is a procedure that can provide critical information regarding lymph node status and the accurate regional staging of the cancer. It provides information specific to the individual patient and so is important to provide appropriate personalized and optimal treatment regimes to eliminate the cancer [6,8-12]. Morton [10] confirmed with the long-term results of the Multicenter Selective Lymphadenectomy Trial that SNB in head and neck melanoma increases both the disease-free and the specific survival of the patient. Active surveillance as a method of managing potential metastasis has been exposed as inadequate in a recent prospective ramomized trial or oral cancer (de Cruz). In case of positive sentinel node biopsy, Andersen et al. [13] indicate that there is a survival benefit for patients who undergo completion of lymph node dissection. Consequently SNB has been advocated in the UK by NICE to be adopted nationally in the management of mouth cancer. The advantage for most patients, is to avoid neck dissection. In the context of patients with cancers in the upper face or scalp if cancer contaminated lymph nodes are allowed to enlarge until clinicaly apparent by a wait and see policy then patients are condemmed to a total parotidectomy frequently with sacrifice of part or all of the facial nerve and a concomitant neck dissection followed by adjuvant RT. This has to be borne by elderly patients in the main resultant poor outcome. From the medico-economic point of view, the SLNB approach appears to be more efficient compared to the traditional surgical approach [14].
The parotid is a common drainage area for cutaneous head and neck tumors in the upper face and scalp. In this small study a range of cutaneous cancer were included because they had the common biological feature of preferential spread via the lymphatic system. In all cases the SN lay in the parotid gland [5]. In a series of cadaver dissection [15] and in a clinical retrospective review [16] the intraparotid nodes were superficial to the facial nerve, and in most cases they were located in the preauricular area or in the parotid tail.
Hancock [17] demonstrated that local dissection is a safe and effective method of removing parotid lesions without threatening the facial nerve. The surgical procedure of extra capsular dissection applied in the current cases is a variation of the aforementioned technique. There was no injury to the facial nerve in this small series of cases which might in part be attributed to the use of IONM. The advantage of this test is the early detection of the facial nerve or one of its peripheral branches [1,18] and is thought to reduce mechanical trauma to the these delicate structures.
Current experience of the application of SNB in head and neck cancer confirms that it is an a reliable way of identifying early lymphatic metastasis and is a safe oncological procedure. The current study demonstrates that by adopting a minimally invasive approach to parotid dissection in conjunction with IONM, that SN can be safely retrieved from the parotid gland. The technique has potentially an important application in elderly individuals with cutaneous cancers of the scalp and temple that are at risk of metastasis. If metastasis are allowed to materialize clinically these patient’s do not tolerate easily the therapeutic regime normally required to cure the disease. This study opens the prospect of the selective use parotid SNB.
We have no conflicts of interest.
We would like to thank Dr. P. Rauch for his data management of the oncologic surgical department.
- Disant F, Dolivet G (2013) Tumeurs de la face et du cou à point de départ cutané. Société Française d'Oto-Rhino-Layngologie et de Chirurgie de la Face et du Cou ed. Cou SFdO-R-LedCdlFed, aris: Société Française d'Oto-Rhino-Layngologie et de Chirurgie de la Face et du Cou.
- Hayashi T, Furukawa H, Oyama A, Funayama E, Saito A, et al. (2012) Dominant lymph drainage in the facial region: evaluation of lymph nodes of facial melanoma patients. Int J Clin Oncol 17: 330-335. [Crossref]
- Medina JE, Ferlito A, Brandwein MS, Fisher SR, Pellitteri PK, et al. (2002) Current management of cutaneous malignant melanoma of the head and neck. Acta Otolaryngol 122: 900-906. [Crossref]
- Nijhawan N, Marriott C, Harvey JT (2010) Lymphatic drainage patterns of the human eyelid: assessed by lymphoscintigraphy. Ophthal Plast Reconstr Surg 26: 281-285. [Crossref]
- George KS, McGurk M (2011) Extracapsular dissection--minimal resection for benign parotid tumours. Br J Oral Maxillofac Surg 49: 451-454. [Crossref]
- Dwojak S, Emerick KS (2015) Sentinel lymph node biopsy for cutaneous head and neck malignancies. Expert Rev Anticancer Ther 15: 305-315. [Crossref]
- Ho VH, Ross MI, Prieto VG, Khaleeq A, Kim S, et al. (2007) Sentinel lymph node biopsy for sebaceous cell carcinoma and melanoma of the ocular adnexa. Arch Otolaryngol Head Neck Surg 133: 820-826. [Crossref]
- Flach GB, Bloemena E, Klop WM, van Es RJ, Schepman KP, et al. (2014) Sentinel lymph node biopsy in clinically N0 T1-T2 staged oral cancer: the Dutch multicenter trial. Oral Oncol 50: 1020-1024. [Crossref]
- Maalouf TJ, Dolivet G, Angioi KS, Leroux A, Genin P, et al. (2012) Sentinel lymph node biopsy in patients with conjunctival and eyelid cancers: experience in 17 patients. Ophthal Plast Reconstr Surg 28: 30-34. [Crossref]
- Morton DL (2012) Overview and update of the phase III Multicenter Selective Lymphadenectomy Trials (MSLT-I and MSLT-II) in melanoma. Clin Exp Metastasis 29: 699-706. [Crossref]
- Schmalbach CE, Bradford CR (2015) Is sentinel lymph node biopsy the standard of care for cutaneous head and neck melanoma? Laryngoscope 125: 153-160. [Crossref]
- Takahashi A, Imafuku S, Nakayama J, Nakaura J, Ito K, et al. (2014) Sentinel node biopsy for high-risk cutaneous squamous cell carcinoma. Eur J Surg Oncol 40: 1256-1262. [Crossref]
- Andersen PS, Chakera AH, Thamsborg AK, Kølle SF, Schmidt G, et al. (2014) Recurrence and survival after neck dissections in cutaneous head and neck melanoma. Dan Med J 61: A4953. [Crossref]
- O'Connor R, Pezier T, Schilling C, McGurk M (2013) The relative cost of sentinel lymph node biopsy in early oral cancer. J Craniomaxillofac Surg 41: 721-727. [Crossref]
- McKean ME, Lee K, McGregor IA (1985) The distribution of lymph nodes in and around the parotid gland: an anatomical study. Br J Plast Surg 38: 1-5. [Crossref]
- Loree TR, Tomljanovich PI, Cheney RT, Hicks WL Jr, Rigual NR (2006) Intraparotid sentinel lymph node biopsy for head and neck melanoma. Laryngoscope 116: 1461-1464. [Crossref]
- Hancock BD (1999) Clinically benign parotid tumours: local dissection as an alternative to superficial parotidectomy in selected cases. Ann R Coll Surg Engl 81: 299-301. [Crossref]
- Upton DC, McNamar JP, Connor NP, Harari PM, Hartig GK (2007) Parotidectomy: ten-year review of 237 cases at a single institution. Otolaryngol Head Neck Surg 136: 788-792. [Crossref]



