Abstract
Gabapentin is an effective agent in reducing postoperative pain and opioid consumption. However, it is unclear when the optimum time of administration is, with in vitro experiments showing a possible pre-emptive effect. Therefore, the aim of this review to evaluate whether gabapentin has a clinically significant pre-emptive analgesic effect in patients undergoing surgery.
We undertook a systematic search of MEDLINE, EMBASE, Cinahl and AMED databases including randomised controlled trials that evaluated preemptive gabapentin versus postincision gabapentin. We included 4 studies with 333 participants. There was no benefit with preemptive administration of gabapentin for 24-hour morphine consumption (MD -0.11mg; -1.59 mg to 1.36 mg; p=0.88) or postoperative pain scores. In addition, there was no difference in the incidence of postoperative opioid and gabapentin side effects. However, current studies may be underpowered for these outcomes.
In conclusion, from the limited data thus far, there appears to be no preemptive analgesic effect with gabapentin and timing of administration should focus on other clinical factors rather than on the basis of any increased clinical efficacy.
Key words
gabapentin, postoperative pain, surgery
Introduction
Postoperative pain is a common consequence of surgery, with an incidence of around 80% [1]. Despite advances in pain management, over a third of these patients suffer severe or extreme pain. Moreover, pain is one of the main concerns of patients undergoing surgical procedures [1]. Although opioid-based analgesia remains the gold standard and most prevalent analgesic used postoperatively [2], it is associated with significant side effects and associated with additional patient concerns [1]. Therefore, alternative pain management strategies have been proposed to treat postoperative pain [3].
Preemptive analgesia involves administration of analgesia prior to surgical incision and has been proposed as an additional strategy to improve postoperative pain [4]. Such timing of administration has the potential to reduce central sensitisation [5] resulting from surgical incision and lead to improved postoperative pain control. Previous meta-analyses have demonstrating conflicting results as to the clinical efficacy of preemptive analgesia [6,7]. However, the results of these reviews are nearly a decade old and new evidence is emerging on the potential of other analgesic agents capable of inducing a preemptive analgesic effect in clinical trials [8].
Gabapentin is an anticonvulsant, which has proven efficacy in neuropathic pain [9]. It exerts its analgesic properties by binding to the α2δ subunit of calcium channels [10], which reduces neurotransmitter release in nerve fibres. A recent meta-analysis [11] has demonstrated that gabapentin reduces postoperative pain and opioid consumption with additional reductions in postoperative nausea, vomiting and pruritus. However, it is not clear whether preemptive administration of gabapentin is superior to postincision administration.
Therefore, the aim of this systematic review and meta-analysis was to summarise the evidence regarding preemptive administration of gabapentin versus postincision administration for postoperative pain in adult patients undergoing surgery.
Methods
This review was produced in accordance with the PRISMA checklist [12]. The review was not registered prior to the literature search. The study search was undertaken in December 2014 by one of the review authors (BD). We searched MEDLINE, EMBASE, CENTRAL, Cinahl and AMED. Search terms included ‘gabapentin’ OR ‘neurontin’ and ‘surgery’. The medical subject heading (MeSH) term ‘SURGICAL PROCEDURES, OPERATIVE’ was exploded and combined with the terms above. In addition, we searched for unpublished trials on clinicaltrials.gov and the ISRCTN registry. Reference lists were searched and Google Scholar used to identify further studies that had cited those included. We contacted authors if further information was required that was not included in the original publication.
The inclusion criteria for the review included randomised controlled trials in any form of surgery. We considered populations of adults only (>18 years old). The preemptive group included participants who were administered gabapentin prior to surgical incision. The comparator included a group who were administered gabapentin after surgical incision. There was no language or publication status restrictions on the studies considered. Two of the study authors (BD and JW) independently evaluated studies against the inclusion criteria and agreement reached by consensus.
Primary outcomes included 24-hour morphine consumption and pain scores at 1, 2, 6, 12 and 24 hours. We regarded reductions in morphine consumption of 10mg and pain scores of 1.5 as clinically significant [13]. Secondary outcomes included opioid and gabapentin side effects as reported by the authors. We converted fentanyl to morphine equivalents using the following ratio: 1:100 [14]. Where pain scores were reported over defined time periods, we included data at the latest time point within the range. Data were extracted onto an electronic database including study name, year, number of participants, percentage female, type of surgery, mean age, country, type of anaesthesia and pain scale used. Internal validity of the included studies was assessed using the Cochrane risk of bias tool [15].
Pain scores and 24-hour morphine consumption are reported as mean differences (MD) using inverse variance. Side effects are reported as Mantel-Haenszel risk ratios (RR). All effect estimates are reported with a 95% confidence intervals (CI) and p value. If results were not reported in the text, we attempted to contact authors. If we received no reply, we extracted data from graphs if necessary. If subgroups were reported, we combined these into one group for the purposes of analysis. If standard deviations were not reported, these we estimated from other studies in the analysis [16].
We used a random effects model due to the significant clinical heterogeneity in the studies. We assessed heterogeneity using the I2 statistic and Cochran’s Q test. We planned to undertake assessment of publication bias using Funnel plots and Egger’s linear regression test. However, the small number of studies precluded this [17]. Similarly, the small number of studies precluded investigation of heterogeneity using meta-regression [18]. We undertook sensitivity analysis removing any studies that received high risk of bias on the risk of bias tool. All analyses were undertaken using Review Manager 5.3 [19].
Results
Following initial searches, 145 studies were identified and underwent full text review (Figure 1). Four studies were identified as matching the inclusion criteria and were included in the final analysis with a total number of participants of 333 [20-23]. Only one study was felt to be at high risk of bias in one domain (Figure 2). All studies were conducted in different types of surgery. The data from two studies [20,22] were not included in the pain score data until after 2 hours, which is when the postincision gabapentin dose was administered.
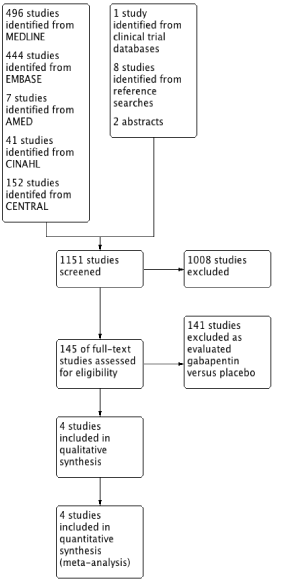
Figure 1. PRISMA flowchart
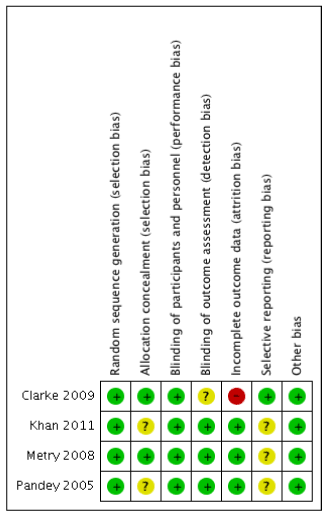
Figure 2. Cochrane risk of bias tool for included studies. Green indicates low risk, yellow indicates unclear risk and red indicates high risk
In terms of primary outcomes, there was no reduction in 24-hour morphine consumption with preemptive gabapentin versus postincision gabapentin in all 4 studies (Figure 3). There was no evidence of statistical heterogeneity (I2=0%; p=0.73). Similarly, there were no reductions in pain scores at any time point postoperatively. One study [23] reported pain scores at 1 hour and these were similar between groups (MD -0.50; 95% CI -1.42 to 0.42; p=0.29). No study reported pain scores at 2 hours. At 6 hours, 2 studies reported this outcome [22,23] with no difference between groups (MD 0.20; 95% CI -0.20 to 0.59; p=0.33). There were also no differences at 12 and 24 hours in 4 studies [20-23] (Figure 4 and 5 respectively). There was no evidence of statistical heterogeneity at any time point (I2=0%; p>0.05).
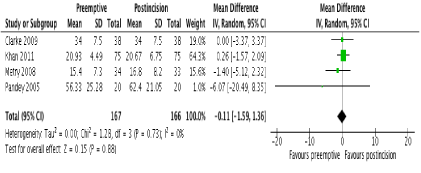
Figure 3. Forest plot of 24-hour morphine consumption
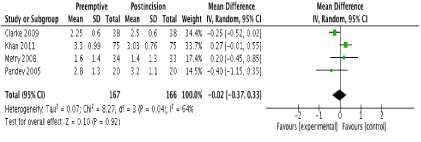
Figure 4. Forest plot of pain scores at 12 hours
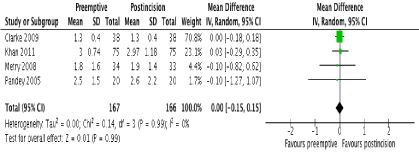
Figure 5. Forest plot of pain scores at 24 hours
In terms of opioid side effects, there was no difference between groups in 3 studies [20,21,23] in the incidence of nausea (RR 1.08; 95% CI 0.64 to 1.84; p=0.77). Four studies reported postoperative vomiting, with no difference between groups (Figure 6). Two studies [20,23] reported postoperative pruritus with no difference between groups (RR 0.66; 95% CI 0.30 to 1.47; p=0.31). There was no evidence of statistical heterogeneity in any of these outcomes (I2=0%; p>0.05).
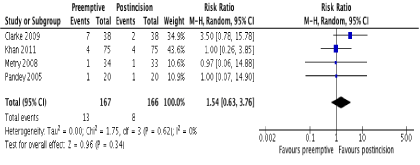
Figure 6. Forest plot of postoperative vomiting
There were no differences found in the incidence of gabapentin side effects between the preemptive and postincision groups. Three studies [20,21,23] reported postoperative dizziness with no difference between groups (RR 0.79; 95% CI 0.39 to 1.57; p=0.49). The same three studies also found no difference in the incidence of postoperative sedation (RR 0.70; 95% CI 0.35 to 1.38; p=0.30). Both outcomes revealed no evidence of statistical heterogeneity (I2=0%; p>0.05).
One study [20] reported chronic pain at 6 months following total hip arthroplasty. They found no differences between the groups in chronic pain (p>0.05). Another study [23] reported a range of other adverse events. There were no statistical differences between the preemptive and postincision groups in any outcomes including fatigue, feeling on a high, lack of concentration and headache. However, incidence of these events was low.
Discussion
This systematic review and meta-analysis has demonstrated that preemptive gabapentin confers no additional benefits in postoperative pain or side effects when compared to the same dose given postincision. Indeed, there were no clinically or statistically significant differences in pain scores, 24-hour morphine consumption or opioid and gabapentin side effects. Although there was a trend towards lower incidences of sedation, pruritus and dizziness and higher incidence of vomiting these differences were not significant.
A recent meta-analysis has demonstrated gabapentin reduces postoperative pain, morphine consumption and opioid side effects when compared to placebo [11]. However, dosing regimes and timing of gabapentin administration were highly heterogeneous, with doses given both pre and postoperatively. Previous research has highlighted potential preemptive analgesic effects with NSAIDS, epidural anaesthesia, local anaesthetic wound infiltration and paracetamol [7,8]. However, the results of this review suggest no preemptive effect with gabapentin, with similar postoperative pain scores and 24-hour morphine consumption.
Despite this, the incidence of postoperative side effects may currently be underpowered to establish any firm conclusions of lack of efficacy. The incidence of side effects in the included studies was low (<20%). In order to demonstrate a difference in side effects from 20% to 10%, a sample size of 400 patients would be required with an a=0.05 and a 1-b=0.8. Larger sample sizes would be needed with the lower incidences observed in some adverse events. Therefore, conclusions from this review on the incidence of side effects are currently limited by a lack of power.
The results of this review contradict those obtained from in vitro experiments with gabapentinoids. Using a rat postoperative pain model, pregabalin (that also binds to the same site as gabapentin) administered before incision resulted in a longer duration of anti-hyperalgesia compared to postincision administration [24]. In another study, administration of intra-thecal gabapentin was more effective when administered before injection of formalin in reducing phase 2 responses when compared to administration after formalin injection [25]. These observations have not been replicated in clinical studies, the reasons for which remain unclear. However, human models have demonstrated that gabapentin may be effective at both preventing development of and reducing established neuronal sensitisation. In a human volunteer study [26], gabapentin was effective at both reducing the development and reducing existing secondary hyperalgesia. These results may help explain why gabapentin was equally effective when given preoperatively or postoperatively.
With regards to timing of gabapentin administration, although this review has found no benefit of preemptive administration in terms of postoperative pain, preemptive administration may have other clinical and logistic advantages. Preemptive gabapentin allows administration with the patient fully alert and capable of swallowing the tablet. Indeed, included studies that gave postincision doses had to do so via an NG tube [21] or two hours after surgery [20,22]. This later dosing may miss important clinically significant reductions in immediate postoperative pain scores found in a recent meta-analysis [11]. Furthermore, the same review found a reduction in preoperative anxiety with gabapentin, an effect that would be missed with postincision administration.
|
Study
|
Mean age
|
Sex
|
N
|
Intervention and Comparator
|
Country
|
Type of anaesthesia
|
Type of surgery
|
Pain Score
|
|
Clarke et al 2009 [20]
|
60.2
|
39%
|
114
|
600mg 2hrs before versus 600mg 2hrs after surgery
|
Canada
|
Spinal anaesthesia
|
Total hip arthroplasty
|
Visual analogue scale (10)
|
|
Khan et al 2011 [21]
|
41.8
|
35%
|
175
|
600mg, 900mg or 1200mg 2hrs before versus immediately post-incision via NG tube
|
Iran
|
General anaesthesia
|
Single level lumbar laminectomy
|
Visual analogue scale (10)
|
|
Metry et al 2008 [22]
|
57.8
|
100%
|
101
|
1200mg 2 hours before versus 1200mg 2 hrs after surgery
|
Egypt
|
General anaesthesia
|
Mastectomy
|
Visual analogue scale (10)
|
|
Pandey et al 2005 [23]
|
2021 Copyright OAT. All rights reserv
43.6
|
68%
|
60
|
600mg 2hrs before versus 600mg post-incision
|
India
|
General anaesthesia
|
Open donor nephrectomy
|
Visual analogue scale (10)
|
Table 1. Characteristics of included studies. Sex reported as percentage female
There are several limitations to this review. Firstly, included randomised controlled trials were clinically heterogeneous in terms of types of surgery, dose of gabapentin used and timing of gabapentin administration. Second, current studies may be underpowered for dichotomous outcomes such as gabapentin and opioid adverse events as alluded to previously. Thirdly, the small number of included studies precluded investigation of heterogeneity or publication bias, which has previously been identified in a meta-analysis of gabapentin [11].
In conclusion, it appears from the limited data thus far, that gabapentin exerts no preemptive effect for postoperative analgesia. Therefore future trials should aim to evaluate these outcomes in order to identify the optimum time of gabapentin administration. Despite this, preemptive doses of gabapentin may have other clinical advantages, which make it a more suitable time for administration.
References
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ (2003) Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg 97: 534-540. [Crossref]
- Benhamou D, Berti M, Brodner G, De Andres J, Draisci G, Moreno-Azcoita M, et al. (2008) Postoperative Analgesic THerapy Observational Survey (PATHOS): a practice pattern study in 7 central/southern European countries. Pain 136: 134-141. [Crossref]
- White PF, Kehlet H (2010) Improving postoperative pain management: what are the unresolved issues? Anesthesiology 112: 220-225. [Crossref]
- Dahl JB, Kehlet H (1993) The value of pre-emptive analgesia in the treatment of postoperative pain. Br J Anaesth 70: 434-439. [Crossref]
- Woolf CJ, Chong MS (1993) Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77: 362-379. [Crossref]
- Møiniche S, Kehlet H, Dahl JB (2002) A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology 96: 725-741. [Crossref]
- Ong CK, Lirk P, Seymour RA, Jenkins BJ (2005) The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg 100: 757-773. [Crossref]
- Doleman B, Read D, Lund JN, Williams JP (2015) Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery: Systematic Review and Meta-Analysis. Regional Anesthesia and Pain medicine 40: 706-712. [Crossref]
- Moore RA, Wiffen PJ, Derry S, McQuay HJ (2011) Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 16: CD007938. [Crossref]
- Rose MA, Kam PC (2002) Gabapentin: pharmacology and its use in pain management. Anaesthesia 57: 451-462. [Crossref]
- Doleman B, Heinink TP, Read DJ, Faleiro RJ, Lund JN, et al. (2015) A systematic review and meta-regression analysis of prophylactic gabapentin for postoperative pain. Anaesthesia 70: 1186-1204. [Crossref]
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 151: 264-269. [Crossref]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, et al. (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9: 105-121. [Crossref]
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E (2001) Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage 22: 672-687. [Crossref]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928. [Crossref]
- Higgins J, Deeks JJ, Altman DG. (2008). Special topics in statistics. In: Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, John Wiley and Sons Ltd. 481-529.
- Sterne JAC, Egger M, Moher D (2008) Addressing reporting biases. In: Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, John Wiley and Sons Ltd. 481-529.
- Deeks JJ, Higgins JPT, Altman DG. (2008) Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, John Wiley and Sons Ltd. 481-529.
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Clarke H, Pereira S, Kennedy D, Andrion J, Mitsakakis N, et al. (2009) Adding gabapentin to a multimodal regimen does not reduce acute pain, opioid consumption or chronic pain after total hip arthroplasty. Acta Anaesthesiol Scand 53: 1073-1083. [Crossref]
- Khan ZH, Rahimi M, Makarem J, Khan RH (2011) Optimal dose of pre-incision/post-incision gabapentin for pain relief following lumbar laminectomy: a randomized study. Acta Anaesthesiol Scand 55: 306-312. [Crossref]
- Metry A, Ishak S, Khattab A (2008) Does gabapentin have preemptive effects in women undergoing mastectomy?. Anaesthesia and Intensive Care in Italy 59: 62-76.
- Pandey CK, Singhal V, Kumar M, Lakra A, Ranjan R, Pal R, et al. (2005) Gabapentin provides effective postoperative analgesia whether administered pre-emptively or post-incision. Canadian Journal of Anaesthesia 52: 827-831. [Crossref]
- Field MJ, Holloman EF, McCleary S, Hughes J, Singh L (1997) Evaluation of gabapentin and S-(+)-3-isobutylgaba in a rat model of postoperative pain. J Pharmacol Exp Ther 282: 1242-1246. [Crossref]
- Yoon MH, Yaksh TL (1999) The effect of intrathecal gabapentin on pain behavior and hemodynamics on the formalin test in the rat. Anesth Analg 89: 434-439. [Crossref]
- Dirks J, Petersen KL, Rowbotham MC, Dahl JB (2002) Gabapentin suppresses cutaneous hyperalgesia following heat-capsaicin sensitization. Anesthesiology 97: 102-107. [Crossref]






