Abstract
Synovial Sarcoma is an aggressive, yet a relatively chemosensitive malignant mesenchymal neoplasm, which displays variable epithelial differentiation, and typically occurs in adolescents and young adults, most frequently in the lower extremities. Synovial Sarcoma is also reported at certain unconventional sites, where its inherent morphologic heterogeneity and overlapping histopathologic features with certain tumors indigenous to that site can lead to a diagnostic dilemma. Presently, the diagnostic gold standard of a synovial sarcoma is demonstration of the characteristic t(X;18) (p11.2; q11.2) translocation, resulting in SYT-SSX fusion, especially in cases occurring at unconventional sites. Larynx is one of the rarely reported sites for a synovial sarcoma, with only 28 such cases reported till date, including only six cases confirmed by molecular testing.
We report three cases of primary laryngeal synovial sarcoma which presented a diagnostic challenge and were confirmed by molecular testing. Although rare, Synovial Sarcoma should be kept as a differential diagnosis of spindle cell neoplasms and biphasic tumors occurring in the larynx. In view of the difference in treatment and patient outcome from the relatively more frequently occurring tumors in the larynx, such as sarcomatoid carcinoma and minor salivary gland neoplasms, its accurate diagnosis with molecular testing in such cases is crucial.
Key words
Synovial sarcoma, larynx, SYT-SSX
Introduction
Synovial sarcoma is an aggressive, yet a relatively chemosensitive malignant mesenchymal neoplasm which displays variable epithelial differentiation on histopathologic examination, and typically occurs in adolescents and young adults, commonly in males [1-3]. Histopathologically, synovial sarcoma is classified as biphasic, monophasic spindle cell, monophasic epithelial or poorly differentiated type, based on the relative prominence of the two cell populations, namely mesenchymal and epithelial, along with the degree of tumor differentiation [1,4]. Despite its morphological heterogeneity, synovial sarcoma is characterized by a unique molecular signature, t(x;18) (p11.2; q11.2) translocation, resulting in the fusion of SS18 (formerly SYT) gene with one of the SSX genes (SSX1, SSX2, and rarely, SSX4) [5]. Identification of this translocation by fluorescence in situ hybridization or reverse-transcriptase polymerase chain reaction is accepted as the diagnostic standard, irrespective of morphologic features and tumor location [3].
Synovial sarcoma can occur at almost any anatomic site, although majority of tumors are reported to arise in the lower extremities [2-4,6]. The head and neck region is an unusual site for this neoplasm, with only 2-10% SSs reported at this location [2,5]. Within the head and neck region, synovial sarcoma originates in the paravertebral soft tissue, and presents as a parapharyngeal mass; hypopharynx is another common location of head and neck synovial sarcoma [2,5,7]. The larynx is an extremely rare site, accounting for 3-10% of all head and neck synovial sarcomas [4]. Due to its rarity, decision-making regarding the surgical procedure, neoadjuvant and adjuvant therapies to be used poses a challenge to the treating otolaryngologist. We therefore describe the clinical, histopathologic and molecular features of primary laryngeal synovial sarcomas diagnosed at our tertiary care centre over a period of eight years, and review cases previously published in literature (Table 1). Differential diagnoses and treatment implications are discussed herewith.
Table 1. Clinicopathological features of published cases of laryngeal synovial sarcoma
Author (year) |
Age/ Sex |
Location |
Histological type |
t(X;18) |
Management |
Follow-up |
Miller (1975) |
23/F |
Interarytenoid area and arytenoids |
- |
- |
Excision; supraglottic laryngectomy; total laryngectomy |
NED at 12 years |
Quinn (1984) |
76/M |
Subglottis |
Biphasic |
- |
Right extended hemilaryngectomy |
NED at 3yrs |
Pruszczynski
(1989) |
28/F |
AEF, false vocal cord |
Monophasic |
|
Local excision, RT (66Gy) |
NED at 3 years |
Ferlito (1991) |
28/M |
AEF, epiglottis |
Biphasic |
- |
Preop. RT (25Gy); supraglottic
laryngectomy; RT (50Gy) |
NED at 16 years |
Morland (1994) |
14/M |
Arytenoids |
- |
- |
Excision; laryngectomy, CT and RT (60Gy) for recurrence |
Recurrence at 3 years; NED 10 months after CT, RT |
Dei Tos (1998) |
27/M |
AEF |
- |
t(X;18) |
Local excision, hemilaryngectomy following recurrence, CT and RT (62Gy) |
recurrence after 3 months, NED after 9 months |
Taylor (2002) |
68/F |
Cricoid cartilage |
- |
- |
Total laryngopharyngectomy |
n/a |
Bilgic (2003) |
24/M |
Epiglottis, AEF, arytenoid |
Biphasic |
- |
Excision; hemilaryngectomy; total laryngectomy, RT (45Gy) for recurrence; CT for metastases |
Local recurrence at 1 year; Lung metastasis after 20 months; NED 3.5 years after CT |
Papaspyrou (2003) |
16/M |
Supraglottic larynx |
Biphasic |
|
CO 2 laser excision, RT |
NED at 2 years |
Szuhai (2004) |
54/M |
Supraglottic larynx |
Monophasic |
t(X;18) by multicolor FISH and RT-PCR |
Laryngopharyngectomy |
NED at 2 years |
Boniver (2005) |
- |
AEF |
- |
- |
CO 2 laser resection |
NED at 3 years |
Abou Zeid (2006) |
26/M |
Supraglottic larynx |
- |
- |
CO2 laser resection |
n/a |
Mhawech-Fauceglia (2007) |
79/ F |
Supraglottic larynx, AEF, superior aspect of glottis |
Biphasic |
t(X;18) by karyotyping and BAP FISH |
Total laryngectomy |
NED at 3 months |
Capelli (2007) |
57/M |
Laryngeal ventricle |
Monophasic |
- |
Laser cordectomy |
NED at 14 months |
Fernández-
Aceñero (2009) |
12/M |
Supraglottic larynx |
- |
- |
Chemotherapy+ Local resection |
NED at 4 months |
Al-Nemer (2011) |
26/M |
Larynx |
- |
- |
Surgery, RT |
NED at 20 months |
Hernandez (2013) |
21/F |
Larynx |
- |
t(X;18) by BAP FISH |
Surgery (R0) |
DOD at 12 months |
Saxby (2013) |
20/M |
AEF; supraglottic larynx, through cricothyroid membrane into thyroid |
Biphasic |
t(X;18) by karyotyping |
Total laryngectomy and hemithyroidectomy; CT (six cycles) followed by RT |
NED at 18 months |
Bao (2013) |
37/M |
AEF, pyriform sinus |
Monophasic |
- |
Partial laryngectomy, RT (66 Gy) ; partial laryngopharyngectomy for recurrence; CT (3 cycles), Nimotuzumab for metastases |
Recurrence at 16 months; second recurrence, lymph node and lung metastases after 9 months |
Luna-Ortiz (2013) |
21/F |
AEF |
Biphasic |
t(X;18) by BAP FISH |
Supraglottic laryngectomy; RT (46 Gy) for recurrence |
Lymph node metastasis at 5 years 4 months; NED at 2 years after RT |
Crowson (2015) |
26/F |
Supraglottic larynx at base of epiglottis |
- |
- |
Extended modified supraglottic laryngectomy, CT |
n/a |
Crowson (2015) |
24/M |
Hypopharynx, larynx, pyriform sinus |
- |
- |
Total laryngectomy, partial pharyngectomy, partial esophagectomy, hemitracheiectomy; RT (64.8 Gy); CT |
n/a |
Crowson (2015) |
34/M |
Supraglottic larynx, pyriform sinus |
- |
- |
Partial supraglottic laryngectomy with partial pharyngectomy and modified neck dissection, RT (68.4 Gy),
CT |
n/a |
Crowson (2015) |
45/M |
Supraglottic larynx |
- |
- |
Transoral laser excision, RT (60 Gy) |
n/a |
Crowson (2015) |
19/F |
Supraglottic larynx |
- |
- |
Total laryngectomy |
n/a |
Javed (2015) |
16/F |
Supraglottic larynx |
Monophasic |
- |
Excision, RT (60 Gy) |
n/a |
Mohammadi (2016) |
23/M |
Epiglottis, AEF, hypopharynx |
Biphasic |
- |
Wide excision, RT |
NED at 42 months |
Guinchard (2016) |
21/F |
Arytenoid |
Monophasic |
- |
Vertical hemilaryngectomy, RT |
NED at 5 years |
AEF – aryepiglottic fold; BAP- break-apart; CT – chemotherapy; DOD – dead due to disease; FISH – fluorescent in situ hybridization; RT – radiotherapy; RT-PCR – reverse transcriptase polymerase chain reaction; NED – no evidence of disease
Material and methods
Cases of primary laryngeal synovial sarcoma diagnosed at our institution over the last eight years (2008-2015) were retrieved from departmental records. Clinical findings and details of treatment and follow-up were collected from patient charts in the electronic medical record system, as well as by telephonic conversation. Patients were followed up every three months for the first six months, and then every six months. Slides and blocks for each case were retrieved from the departmental archives. Hematoxylin and eosin stained slides, as well as immunohistochemical stained microsections were reviewed. Molecular testing for SS18-SSX fusion was performed as described previously [8] Approval was obtained from the Institutional Ethics Committee to conduct this study.
Results
Three cases of primary laryngeal synovial sarcoma were diagnosed during the eight year period (Table 2). These included two cases that were registered at our hospital for treatment, and one case where biopsy material was received for a second opinion. Patients included one female and two males, all in the fourth decade of life. All three patients had non-specific symptoms, such as difficulty in swallowing and change in voice. There was no history of smoking or alcohol consumption. Clinical presentation, tumor location, treatment received and follow-up are summarized in Table 2.
Table 2. Clinical features of three cases of laryngeal synovial sarcoma in present series
Patient no. |
Age/ Sex |
Presenting features |
Location |
Treatment received |
Histopathological diagnosis |
RT-PCR |
Follow up |
Patient 1 |
32 years/ Female |
Dysphagia x 1 year |
Pedunculated mass in posterior wall of hypopharynx, pyriform fossa and aryepiglottic fold |
Wide excision |
Biphasic synovial sarcoma |
SYT-SSX2 |
NED at 7.5 years |
Patient 2 |
31 years/ Male |
Hoarseness of voice x 2 months |
Proliferative growth in right pyriform fossa, glottis, aryepiglottic fold, post-cricoid area |
Transoral CO2 laser resection (R2); Total laryngectomy one month later; No adjuvant therapy |
Biphasic synovial sarcoma |
SYT-SSX1 |
NED at 5.5 years |
Patient 3 |
35 years/ Male |
Hoarseness of voice x 1 month |
Proliferative growth in left pyriform fossa |
Wide excision; no adjuvant therapy |
Biphasic synovial sarcoma |
SYT-SSX1 |
NED at 1.5 years |
On microscopic examination, all three cases showed features of a biphasic tumor, comprising mesenchymal and epithelial components (Figure 1). The mesenchymal component was prominent in tumors from Patients 2 and 3, essentially consisting of compactly arranged, uniform, oval to short spindle cells having scant amphophilic cytoplasm, and nuclei with coarse chromatin and inconspicuous nucleoli. Focal epithelial component in the form of cleft-like glands and tubules, imperceptibly blending with the mesenchymal component, was present in these cases. In Case 1, however, the epithelial component was predominant, in the form of papillary and glandular structures lined by cuboidal cells with abundant cytoplasm, large vesicular nuclei displaying moderate atypia, prominent nucleoli and mitotic figures. Only a thin rim of the mesenchymal component, composed of spindle shaped cells surrounding the epithelial structures, was identified. The mesenchymal component in Cases 2 and 3 showed moderate nuclear pleomorphism, and mitotic counts ranging from 6-8/10 high power fields. Necrosis or lymphovascular emboli were not seen.
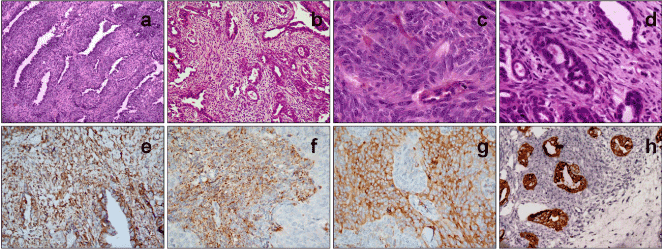
Figure 1. Photomicrographs from Case 2 show a biphasic tumor with cleft-like glands (a; HE, x100) and tubular structures (b; HE, x100) interspersed in a background of monomorphic spindled cells with scant cytoplasm and ovoid nuclei having fine chromatin and inconspicuous nucleoli (c; HE, x400); epithelial structures are lined by cuboidal cells with eosinophilic cytoplasm and pleomorphic nuclei (d; HE, x400). Immunohistochemistry shows diffuse CD99 positivity (e; IHC, x200), while bcl2 (f; IHC, x200) and calponin (g; IHC, x200) are positive in the spindle cell component and pancytokeratin decorates the epithelial component (h; IHC, x200).
Immunohistochemistry (Figure 1 e-h) was performed in Cases 1 and 3 only, as there was insufficient tissue in the paraffin block in Case 2. Tumor cells showed cytoplasmic positivity for CD99 in both, the epithelial and mesenchymal components, whereas diffuse positivity for bcl2 and calponin was seen mostly in the mesenchymal component. Pancytokeratin (AE1/AE3) and high molecular weight cytokeratin were focally positive in both the cases, highlighting the epithelial component, whereas S-100 and CD10 were completely negative. In addition, tumor cells in Case 1 also displayed immunopositivity for CK7. Reverse-transcriptase polymerase chain reaction revealed SS18-SSX1 fusion transcript in Cases 2 and 3 (Figure 2) and SS18-SSX2 fusion transcript in Case 1.
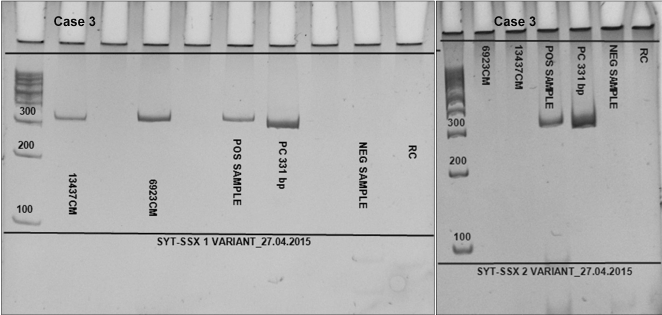
Figure 2. RT-PCR analysis showing t(X; 18) SS18-SSX1 fusion in Case 3, while SS18-SSX2 fusion is absent
Discussion
Tumors arising primarily in the larynx are almost exclusively of epithelial origin, with non-epithelial tumors, including sarcomas, accounting for approximately 1-3% of cases [9]. Primary laryngeal synovial sarcoma is an extremely uncommon entity, with only 28 cases reported till date, of which only six were confirmed by genetic testing (Table 1). [2,3,6,10-15] Herein, we report three additional cases of primary laryngeal synovial sarcoma, all confirmed by molecular testing, which constitutes as the first documentation of these tumors from our subcontinent (Table 2). The mean age of the patients in our series was 32.7 years, with male preponderance, as previously reported [7].
Histopathologically, a classical biphasic synovial sarcoma located in the extremities usually does not pose a diagnostic difficulty, in contrast to a monophasic spindle cell type. However, a low index of suspicion for this tumor arising primarily in the larynx leads to possibility of various differential diagnoses, as in our cases. In Cases 2 and 3, differential diagnoses of sarcomatoid carcinoma, synovial sarcoma and glandular malignant peripheral nerve sheath tumor were entertained, while in Case 1 the possibilities of epithelial-predominant SS and malignant tumor of salivary gland origin were considered. In fact, Case 1 had initially been diagnosed elsewhere as a salivary gland neoplasm, due to the prominent glandular component. As squamous cell carcinoma is the most frequent primary malignancy in the larynx, it is noteworthy that before diagnosing a sarcoma at this location, the possibility of sarcomatoid variant of squamous cell carcinoma should be excluded. Another pitfall is that a hemangiopericytomatous vascular pattern, commonly seen in synovial sarcoma, may often be seen in sarcomatoid squamous cell carcinoma, as well as in spindle cell myoepithelial carcinoma. Glandular malignant peripheral nerve sheath tumor is another mesenchymal tumor that displays focal epithelial differentiation. In such situations, application of an optimal panel of immunohistochemical markers, including demonstration of co-expression of epithelial and mesenchymal markers, is useful for considering diagnosis of synovial sarcoma. At the same time, similar immunohistochemical profile can be noted in sarcomatoid variant of squamous cell carcinoma, as well as in certain tumors of salivary gland origin, such as spindle cell myoepithelial carcinoma which may display immunopositivity for calponin, CD99 and bcl2 in addition to CD10 and S-100 [6]. In addition, both these neoplasms may show complete negativity for epithelial markers, causing them to be easily mistaken for monophasic synovial sarcoma. Therefore, finally, demonstration of either the characteristic SS18 rearrangement by fluorescent in situ hybridization, or demonstration of SS18-SSX fusion transcript by reverse-transcriptase polymerase chain reaction is imperative for confirming the diagnosis of synovial sarcoma [16].
In terms of the specific subtypes of the chimeric transcripts, some investigators have noted that SS18–SSX1 fusion is more frequently associated with biphasic synovial sarcoma, higher proliferation rate, and worse clinical outcome, while SS18-SSX2 fusion is seen predominantly in female patients, in monophasic synovial sarcoma and is associated with a relatively better outcome [17]. Although all the three tumors in our series were biphasic synovial sarcomas, we noted that Case 1 from a 32-year-old female patient, with predominant epithelial component on histology, displayed SS18-SSX2 fusion. At the same time, the number of cases in our series is too small and mean follow-up is not long enough to definitively compare association between subtypes of fusion transcript and clinical outcome.
Therapeutically, surgical resection with clear margins is the mainstay of treatment in cases of head and neck synovial sarcoma. Depending upon tumor size, laryngopharyngectomy, laryngectomy (total or partial), or wide resection of tumor with CO2 laser is performed. All our patients underwent surgical resection. Upfront dissection of neck lymph nodes is not recommended, underlining the importance of distinguishing a synovial sarcoma from sarcomatoid carcinoma on a pre-operative biopsy [11]. The role of neoadjuvant chemotherapy remains questionable at present [13]. Regarding adjuvant therapies, Bilgic et al. treated an operated case of laryngeal synovial sarcoma with distant metastases to lung with post-op radiotherapy followed by chemotherapy, which proved to be effective and the metastatic nodules disappeared [10]. Bao et al. reported the management of local recurrence and distant metastases of a laryngeal synovial sarcoma case with radiation and chemotherapy; the patient however, succumbed to the disease [11]. Thus, the role of adjuvant chemotherapy and radiotherapy is controversial, but these treatment modalities can be offered to patients with incomplete resection and distant metastases.
The outcome of head and neck synovial sarcoma is similar to that of synovial sarcoma of the extremities, with 5-year overall survival rate of 40% to 70% [2,5]. Local recurrences and distant metastases, seen in 21% to 45%, and 18% to 40% of head and neck synovial sarcoma, respectively, remain the main concern in these patients [4,13]. Of the 20 laryngeal synovial sarcomas in literature whose follow up was available, two patients developed local recurrence, two had recurrence as well as distant metastases, one had distant metastases without local recurrence, one died due to disease and fourteen showed no evidence of disease at last follow up (Table 1). Those with recurrence/metastases included monophasic as well as biphasic tumors. While monophasic and biphasic histological types have diagnostic connotations, they have no known impact on patient outcome. All three patients in our series were disease-free after surgical excision at a median follow-up of 5.5 years. Multivariate analyses have shown that clinicopathological features such as size > 5 cm, greater TNM stage at presentation, positive surgical margins, and presence of poorly differentiated components are associated with shorter survival [2,5]. In addition, patients older than 25 years of age, tumors showing capsular invasion, and proliferation index >10% have also been reported to be associated with poorer outcome and greater risk for distant metastasis [3]. All our patients were in the fourth decade of life, had tumors <5 cm in size, negative surgical margins, and lacked poorly differentiated areas. Currently, no specific risk factors for laryngeal synovial sarcoma have been identified. As most cases of laryngeal synovial sarcoma in literature have been reported as isolated case reports, with short follow-up, a meaningful comparison and statistical analysis is not possible.
Conclusion
Synovial sarcoma arising in the larynx is extremely rare, but should be kept in differential diagnosis of spindle cell neoplasms and biphasic tumors arising at this site. As it differs in treatment and patient outcome from the more frequent laryngeal epithelial tumors, arriving at the correct diagnosis preoperatively is essential. While immunohistochemistry is supportive of the diagnosis and serves as a valuable adjunct to histomorphology, reverse-transcriptase polymerase chain reaction for demonstration of SS18-SSX translocations is the gold standard and imperative for confirmation of diagnosis at unusual locations. However, a high index of suspicion is necessary to order the right immunohistochemical panel and translocation studies. Lastly, while early diagnosis and surgery can be curative, patients with laryngeal synovial sarcoma should be kept on long term follow-up, due to the propensity for local recurrence and distant metastases.
References
2021 Copyright OAT. All rights reserv
- Suurmeijer AJH, de Bruijn D, van Kessel AG, Miettinen MM (2014) Synovial sarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC. 213-215.
- Salcedo-Hernández RA, Lino-Silva LS, Luna-Ortiz K (2013) Synovial sarcomas of the head and neck: comparative analysis with synovial sarcoma of the extremities. Auris Nasus Larynx 40: 476-480. [Crossref]
- Javed N, Iqbal J (2015) Synovial sarcoma of the larynx. J Ayub Med Coll Abbottabad 27: 729-730. [Crossref]
- Wushou A, Miao XC (2015) Tumor size predicts prognosis of head and neck synovial cell sarcoma. Oncol Lett 9: 381-386. [Crossref]
- Mallen-St Clair J, Arshi A, Abemayor E, St John M (2016) Factors Associated With Survival in Patients With Synovial Cell Sarcoma of the Head and Neck: An Analysis of 167 Cases Using the SEER (Surveillance, Epidemiology, and End Results) Database. JAMA Otolaryngol Head Neck Surg 142: 576-583. [Crossref]
- Saxby C, Bova R, Edwards M (2013) Laryngeal synovial sarcoma: a rare clinical entity. Case Rep Otolaryngol 2013: 578606. [Crossref]
- Pai S, Chinoy RF, Pradhan SA, D'Cruz AK, Kane SV, et al. (1993) Head and neck synovial sarcomas. J Surg Oncol 54: 82-86. [Crossref]
- Rekhi B, Jambhekar NA, Desai SB, Basak R, Puri A, et al. (2008) A t(X; 18) SYT-SSX2 positive synovial sarcoma in the pelvis of a young adult male: a rare case report with review of literature. Indian J Cancer 45: 67-71. [Crossref]
- Karatayli-Ozgursoy S, Bishop JA, Hillel AT, Akst LM, Best SR (2016) Non-epithelial tumors of the larynx: a single institution review. Am J Otolaryngol 37: 279-285. [Crossref]
- Bilgic B, Mete O, Oztürk SA, Demiryont M, Keles N, et al. (2003) Synovial sarcoma: a rare tumor of larynx. Pathol Oncol Res 9: 242-245. [Crossref]
- Bao YY, Wang QY, Zhou SH, Zhao K, Ruan LX, et al. (2013) Poor outcome of comprehensive therapy in a case of laryngeal synovial sarcoma. Radiol Oncol 47: 111-118. [Crossref]
- Luna-Ortiz K, Cano-Valdez AM, da Cunha IW, Mosqueda-Taylor A (2013) Synovial sarcoma of the larynx treated by supraglottic laryngectomy: case report and literature review. Ear Nose Throat J 92: E20-26. [Crossref]
- Crowson MG, Lalich I, Keeney MG, Garcia JJ, Price DL (2015) Clinicopathologic factors and adjuvant treatment effects on survival in adult head and neck synovial cell sarcoma. Head Neck 37: 375-380. [Crossref]
- Mohammadi G, Khansarinia A (2016) Synovial Sarcoma- A Rare Tumor of the Larynx. Iran J Otorhinolaryngol 28: 233-236. [Crossref]
- Guinchard AC, Monnier P, Jaquet Y, Monnier Y, Ikonomidis C (2016) Modified technique of functional vertical hemilaryngectomy for cancer invading 1 hemicricoid. Head Neck. [Crossref]
- Agarwal AP, Shet TM, Joshi R, Desai SB, Chinoy RF (2009) Monophasic synovial sarcoma of tongue. Indian J Pathol Microbiol 52: 568-570. [Crossref]
- Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, et al. (2002) Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi-institutional retrospective study of 243 patients. Cancer Res 62: 135-140. [Crossref]


