Aim: We evaluated the effectiveness of the renal sympathetic denervation (RSD) in diastolic heat and renal functions, in resistant hypertensive CKD patients.
Methods and results: Data were obtained at baseline and monthly until 6th month of follow-up. Twenty-six out of the 45 patients had LVH and nineteen did not present LVH. The LV mass index decreased from 123.70 ± 38.44 g/m2 at baseline to 106.50 ± 31.88 g/m2 at the 6th month after RSD, P<0.0001. The comparison between baseline and the 6th month post RSD showed that the mitral valve E deceleration time shortened from 242.40 ± 45.11 ms to 214.50 ± 36.40 ms (P<0.0001), and a significant reduction in the isovolumic relaxation time (IVRT) was noted from 126.10 ± 26.56 ms to 107.80 ± 24.53 ms (P<0.0001). Tissue Doppler imaging revealed a significant reduction in the ratio of mitral inflow velocity to annular relaxation velocity (lateral E/e’), a marker of LV diastolic filling pressure, significantly decreased from 9.49 ± 2.57 at baseline to 6.88 ± 1.96 at the 6th month post procedure (P<0.0001). When the variation between baseline and the 6th month post RSD in LVH patients and non LVH patients were compared to the same parameters: mitral valve E deceleration time (-29.65 ± 15.80 ms and -25.53 ± 20.04 ms, respectively, P=0.4439), the IVRT (-20.00 ± 13.78 ms and -16.05 ± 12.65 ms, respectively, P=0.3316), and mitral valve lateral E/e’ ratio (-2.82 ± 1.21 and -2.32 ± 1.27, respectively, P=0.1900) no significant difference was found.
Conclusion: The RSD showed an improvement of diastolic echocardiographic parameters in LVH and non LVH CKD refractory hypertensive patients.
chronic kidney disease, resistant hypertension, renal sympathetic denervation, left ventricular hypertrophy, diastolic echocardiographic parameters
There is a progressive increase in the prevalence of left ventricular hypertrophy (LVH) when the estimated glomerular filtration rate (eGFR) decreases [1-4]. In addition, among participants with more advanced kidney disease on dialysis, magnetic resonance imaging (MRI) with contrast demonstrates a diffuse pattern image with gadolinium uptake suggestive of fibrosis and non-ischemic cardiomyopathy [5]. Nowadays, the ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity (E/e′ ratio) is used for the evaluation of left ventricular filling pressure, and it has been used as a marker to diagnose diastolic heart failure (HF) [6,7]. In hypertensive patients with elevated E/e′ ratio the annual mortality rate is 10% and the ratio is considered to be a prognostic factor for the development of cardiovascular disease (CVD) [8]. Diastolic HF is an important factor that increases mortality related to the cardiovascular system in patients with chronic kidney disease (CKD) whose extent of kidney function deterioration differs [9]. As a non-invasive method to allow early assessment, the E/e’ ratio estimated by tissue Doppler imaging can predict mortality and cardiovascular events in CKD patients with diastolic dysfunction.
Sympathetic hyperactivity is well known to increase cardiovascular risk in CKD patients, and is a hallmark of essential hypertensive state that occurs early in the clinical course of the disease [10-12]. In CKD, the sympathetic hyperactivity seems to be expressed at the earliest clinical stage of the disease, showing a direct relationship with the severity of the condition of renal impairment [13-17]. The interruption of sympathetic hyperactivity and feedback of the renin-angiotensin-aldosterone system cycle may be beneficial for this population. Based on these pathophysiological mechanisms, renal sympathetic denervation (RSD) in CKD patients with resistant hypertension may change left ventricular mass and diastolic parameters, being effective in preventing the evolution to diastolic heart failure.
In this study, we conducted a prospective, longitudinal study in 45 patients with refractory hypertension and CKD stages 2, 3, and 4 who underwent percutaneous RSD. The Committee of Ethics in Research of the Medical School of Universidad Federal Fluminense approved the study and an informed consent was signed by all patients.
Study subjects
This study was conducted in the state of Rio de Janeiro, Brazil as a partnership of Universidade Federal Fluminense and the Hospital Regional Darcy Vargas. Patients were recruited from June 2011 to February 2015 and were derived from the university hospital and the public health network of the county. Patients who had the combination of the following criteria were consecutively enrolled: (i) office systolic blood pressure ≥160 mmHg (or ≥150 mmHg for patients with type 2 diabetes mellitus), confirmed by multiple measurements, despite treatment with non-pharmacological measures and use of at least three antihypertensive drugs (including a diuretic) on maximally tolerated doses or confirmed intolerance to medications; (ii) glomerular filtration rate estimated by the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, eGFR [18] between 15 and 89 mL/min/1.73 m2 (patients with eGFR>60 mL/min/1.73m2 were required to have microalbuminuria); and (iii) age from 18 to 70 years.
Exclusion criteria were pregnancy, valvular heart disease with significant hemodynamic consequences; use of warfarin, stenotic valvular heart disease for which the reduction in blood pressure (BP) could be dangerous; acute myocardial infarction, unstable angina, stroke, or transitory ischemic attack within the previous 6 months; Reno vascular anomalies (including renal artery stenosis, angioplasty with or without stenting, or double or multiple main arteries in the same kidney); and diabetes mellitus type 1 or other secondary cause for hypertension.
All patients involved in this study were already treated for hypertension for at least a year. Baseline medication was unchanged for at least 3 months before renal nerve ablation.
Transthoracic echocardiography
Transthoracic echocardiography was performed at baseline and at the 6th month of follow-up using a GE ultrasound system (Vivid I, General Electric, Frankfurt, Germany) equipped with a multifrequency transducer and tissue Doppler imaging software according to the Guidelines of the American Society of Echocardiography [15]. Data were analyzed and interpreted by 2 experienced echocardiographers.
The left ventricular (LV) mass was calculated from LV linear dimensions using the Devereux formula [19,20]. LV mass was indexed to the body surface area [19,21], as indicated. LVH was considered present when the LV mass exceeded 115 g/m2 for men and 95 g/m2 for women [19].
The diastolic parameters were evaluated accordingly the recommendations for the evaluation of left ventricular diastolic function by echocardiography, described by Nagueh et al. [22].
Study procedures and assessment
In this study, we treated 45 patients (26 men and 19 women) with CKD (stages 2, 3, and 4), and grade 2 and 3 systemic arterial hypertensions. Patients underwent a complete medical history and physical examination. Hypertension was diagnosed on the basis of the current Brazilian Society of Cardiology guidelines and of the current European Society of Cardiology guidelines, for the management of arterial hypertension [23,24]. Patients had previously been screened for secondary forms of hypertension according to current guidelines [23,24]. All the patients underwent history and physical examination, and the antihypertensive medication was reviewed. The BP measurements were performed in the stand, sitting, and supine positions on at least two subsequent visits in both arms. Patients also were submitted to blood sampling for entire blood count and biochemistry (including serum creatinine to estimate GFR). Urine samples were obtained for determination of albuminuria, protein, and creatinine. Twenty-four-hour ambulatory blood pressure monitoring (ABPM), echocardiogram, and Echo Doppler evaluation of the anatomy of the renal arteries of patients were also performed.
To evaluate the true effects of RSD on BP and additional measures, baseline medication was unchanged for at least 3 months before renal nerve ablation and treatment was maintained at follow-up. The patients were instructed not to change the medications and dosages after the procedure unless clinically indicated. Drug records and adherence of each patient were comprehensively reviewed and documented at each visit.
All the patients received i.v. sodium bicarbonate (3 mL/kg) and 0.9% saline for 1 h, as prophylaxis for attenuation of iodinated contrast media-associated nephrotoxicity [25,26]. The procedures were performed in the catheterization laboratory with direct visualization using fluoroscopy and radiopaque contrast. In several cases, we also used three-dimensional mapping system EnSite Velocity (St. Jude Medical, St. Paul, Minnesota, USA) for the construction of renal arteries and aorta anatomy, as well as for radiofrequency application in the selected sites. Under the supervision of an anesthesiologist, patients were pretreated with diazepam or midazolam. Catheterization of the femoral artery by the standard Seldinger technique was performed after s.c. injection of local anesthetic in the inguinal region. A 12-Fr valved sheath was introduced into this artery and unfractionated heparin was administered as i.v. bolus, targeting an activated coagulation time (ACT) > 250 s in the first 10 min. During the procedure, the ACT targeted range was 250-350 s. Subsequently, using an 11-F steerable long sheath (Agilis®, St. Jude Medical, St. Paul, Minnesota, USA) by the standard “over the wire” technique, an angiogram of the aorta and renal arteries were performed, and the 7-Fr ablation catheter with open irrigated tip was inserted (AlCath Flux extra Gold Full Circle 2708; VascoMed GmbH, Binzen, Germany or Therapy™ Cool Path™, St. Jude Medical, St. Paul, Minnesota, USA) inside the renal artery, allowing the delivery of RF energy to the renal artery innervation. Because the application of RF is usually very painful, fentanyl was intravenously administered before the procedure. Radiofrequency applications were performed within the main stem of the renal arteries, bilaterally, with a series of applications with 8W power, 60 s duration each, with an irrigation flow rate of 17 mL/min, aiming > 4 RF applications per renal artery, according to their length. These points ablated were made with at least 5 mm distance between them and moving the catheter from the distal to the proximal in circumferential manner. The number of lesions per artery was chosen based on the artery length measurement by baseline angiography. For arteries, shorter than 20 mm, a minimum of four lesions were applied, and for every increase in 5 mm length one additional lesion was provided. After the procedure, the anatomy of the renal arteries was checked by angiography to assess whether there were any complications during the procedure. At the end of procedure, patients were submitted to another infusion of sodium bicarbonate (1 mL/kg/h) for 6 h [25,26].
After the procedure, patients remained hospitalized for a period of 24 h in a ward. The follow-up was performed weekly for the first month and monthly from the second to the sixth month. In every visit to the office, BP was measured after standing for 10 min in both upper limbs in sitting and supine positions, being considered for the study the mean of four measures. For every change in patient position (standing, sitting, and supine), there was a pause of 5 min. Samples were collected for blood and urine tests to monitoring the variables on the 1, 3, and 6th months. Ambulatory blood pressure monitoring was performed on the first, third, and sixth month after the procedure to evaluate the BP and effectiveness of RSD. Echo Doppler was also performed to evaluate the anatomy of the renal arteries of patients, at first and sixth month after the RSD. Echocardiogram was performed 6 months’ post procedure. The following variables were monitored during the follow-up period: echocardiographic parameters, systolic and diastolic BP, number, and doses of antihypertensive medications, eGFR, and albuminuria.
Statistical analysis
The results were expressed as mean and standard deviation (mean ± SD) of the mean in case of normal distribution and as the median with inter-quartile range otherwise. Statistical tests were all two-sided. Comparisons between two-paired values were performed by the paired t-test in case of Gaussian distribution or, alternatively, by the Wilcoxon test. Comparisons between more than two-paired values were performed by ANOVA for repeated measures or with Kruskal-Wallis ANOVA as appropriate complemented by a post hoc test. Frequencies were compared with x2 test. P-values<0.05 were considered significant. Correlations between two variables were performed by Pearson in case of Gaussian distribution or, alternatively, with the Spearman correlation test. All statistical analysis was performed using the program Graph Pad Prism v 6.0 (Graph pad software, La Jolla, CA, USA).
Baseline characteristics of patients
General features of the 45 patients are listed in Table 1. Twenty-two out of the 45 patients were on stage 2 of CKD, sixteen on stage 3, and seven on stage 4. The mean systolic/diastolic arterial pressure was 180 ± 17/109 ± 12 mmHg. Patients were taking an average of 4.7 ± 1.2 classes of antihypertensive drugs. Twenty-six out of the 45 patients had LVH and nineteen did not present LVH.
Table 1. General features of patients at baseline
|
Overall |
LVH |
Non LVH |
P value
LVH vs. Non LVH |
N |
45 |
26 |
19 |
|
Age (years) |
53.9 ± 11.3 |
56.4 ± 10.5 |
50.4 ± 11.4 |
0.0751 |
Body mass index, kg/m2 |
30.2 ± 4.3 |
29.8 ± 4.0 |
30.9 ± 4.0 |
0.4038 |
Male sex (%) |
26 (58%) |
14 (54%) |
11 (58%) |
1.0000 |
Ethnicity (white) (%) |
35(78%) |
18(69%) |
17(89%) |
0.1536 |
Coronary artery disease (%) |
6(13%) |
2(8%) |
4(21%) |
0.3768 |
Atrial fibrilation (%) |
2(4%) |
2(8%) |
0(0%) |
0.5010 |
Stroke (%) |
6(13%) |
6(23%) |
0(0%) |
0.0316 |
Type 2 diabetes (%) |
15(33%) |
11(42%) |
4(21%) |
0.2027 |
Number of antihypertensives |
4.7 ± 1.2 |
4.7 ± 1.1 |
4.6 ± 1.3 |
0.4047 |
eGFR, mL/min/1.73 m2 (CKD-EPI) |
57.3 ± 22.0 |
53.8 ± 22.0 |
62.1 ± 21.5 |
0.2269 |
Stages of CKD |
|
|
|
|
2 |
22(49%) |
11(42.3%) |
11(57.9%) |
0.3726 |
3 |
16(35.5%) |
10(38.4%) |
6(31.6%) |
0.7565 |
4 |
7(15.5%) |
5(19.3%) |
2(10.5%) |
0.6808 |
Office blood pressure, mmHg |
180 ± 17/109 ± 12 |
181 ± 19/106 ± 11 |
179 ± 16/114 ± 13 |
0.6291/0.0257` |
a Mean ± SD; eGFR. Estimated glomerular filtration rate; LVH, left ventricular hypotrophy; N, number of patients
Efficacy in blood pressure reduction, changes in antihypertensive medications and in renal function after the procedure
These changes (∆) between baseline and 6 months after RSD in office BP, 24-h ABPM, number of antihypertensive drugs, percentage of medication use by class (Table 2), creatinine values, eGFR and albumin:creatinine ratio (ACR) in overall patients, LVH patients and non LVH patients can be better appreciated in Table 3.
Table 2. Medication use, by class, during the study
Types of antihypertensive agents |
Baseline, n=45 (%) |
6th month, n=45 (%) |
Diuretic |
45 (100%) |
36 (80%) |
Aldosterone antagonist |
15 (33%) |
8 (18%) |
Angiotensin-receptor blocker |
38 (84%) |
30 (67%) |
ACE inhibitor |
7 (16%) |
4 (9%) |
Direct renin inhibitor |
3 (10%) |
2 (7%) |
β blocker |
30 (67%) |
25 (56%) |
Calcium-channel blocker |
41 (91%) |
31 (69%) |
Centrally acting sympatholytic |
19 (42%) |
8(18%) |
Vasodilator |
4 (13%) |
0 (0%) |
α-1 adrenergic blocker |
1 (3%) |
0 (0%) |
ACE= angiotensin-converting enzyme.
Table 3. Variation (Δ) of parameters between baseline and 6th month after renal sympathetic denervation
Variable |
Overall (n=45) |
P value |
LVH (n=26) |
P value |
Non LVH (n=19) |
P value |
Office Systolic BP, mmHg |
-43.4 ± 17.2a |
<0.0001 |
-40.9 ± 18.0 |
<0.0001 |
-46.7 ± 16.0 |
<0.0001 |
Office Diastolic BP, mmHg |
-19.2 ± 12.4a |
<0.0001 |
-15.8 ± 11.2 |
<0.0001 |
-23.9 ± 12.6 |
<0.0001 |
Systolic ABPM, mmHg |
-19.4 ± 10.6a |
<0.0001 |
-18.7 ± 10.1 |
<0.0001 |
-20.3 ± 11.4 |
<0.0001 |
Diastolic ABPM, mmHg |
-9.8 ± 10.7a |
<0.0001 |
-8.6 ± 10.7 |
0.0004 |
-11.4 ± 10.8 |
0.0002 |
Number of AH drugs |
-1.1 ± 1.2a |
<0.0001 |
-1.0 ± 1.2 |
0.0002 |
-1.3 ± 1.2 |
0.0003 |
Creatinine values, mg/dl |
-0.24 ± 0.25a |
<0.0001 |
-0.25 ± 0.31 |
0.0005 |
-0.24 ± 0.15 |
<0.0001 |
eGFR, ml/min/1.73m2 |
-15.7 ± 14.1a |
<0.0001 |
-13.5 ± 13.7 |
<0.0001 |
-18.7 ± 14.4 |
<0.0001 |
Albumin: creatinine ratio, mg/g |
-38.4b
(-76.4 to -23.4) |
<0.0001 |
-42.2
(-84.8 to -20.7) |
<0.0026 |
-37.9
(-66.5 to -27.3) |
0.0002 |
aMean ± SD; bMedian (Interquartile range); eGFR, estimated glomerular filtration rate; LVH, left ventricular hypertrophy; Δ, variation.
Changes of echocardiographic parameters
Besides, the LV mass index decreased from 123.70 ± 38.44 g/m2 at baseline to 106.50 ± 31.88 g/m2 at the 6th month after RSD, P<0.0001. The regression of LV mass in patients who underwent RSD was accompanied by an improvement of diastolic functional parameters. The comparison between baseline and the 6th month post RSD showed that the mitral valve E deceleration time (Figure 1A) shortened from 242.40 ± 45.11 ms to 214.50 ± 36.40 ms (P<0.0001), and a significant reduction in the isovolumic relaxation time (IVRT) (Figure 1B) was noted from 126.10 ± 26.56 ms to 107.80 ± 24.53 ms (P<0.0001). Tissue Doppler imaging revealed a significant reduction in the mitral valve E Vmax from 76.96 ± 21.77 cm/s to 71.21 ± 20.70 cm/s (P<0.0001), in the mitral valve A Vmax from 81.82 ± 20.24 cm/s to 71.64 ± 19.70 cm/s (P<0.0001), in the mitral valve E/A ratio from 0.94 ± 0.09 to 0.10 ± 0.16 (P=0.0007), an increase of the diastolic relaxation velocity of the lateral mitral annulus measured by the mitral valve lateral e’ from 8.16 ± 0.95 cm/s to 10.42 ± 1.16 cm/s (P<0.0001). Furthermore, the ratio of mitral inflow velocity to annular relaxation velocity (lateral E/e’), a marker of LV diastolic filling pressure, significantly decreased from 9.49 ± 2.57 at baseline to 6.88 ± 1.96 at the 6th month post procedure (P<0.0001), as shown in Figure 1C. The number of patients (%) with normal LV filling pressures, i.e., an E/e’ ratio ≤ 8, increased from 18 (40%) at baseline to 31 (69%) at 6 months after the RSD, whereas the percentage of patients with increased LV filling pressures based on the E/e’ ratio (E/e’ ratio ≥12) declined from 12 (27%) at baseline to 0 (0%) at the 6th month of follow-up [22]. Consistent with a pronounced reduction in LV filling pressures by RSD, left atrial diameter (LAD) significantly decreased from 40.87 ± 5.91 mm at baseline to 38.53 ± 6.10 mm at the 6th month post procedure, P<0.0001, as shown in Figure 2. Thirty-six patients (80%) had decrease of LAD post procedure. Twenty-three LVH patients (88%) showed this reduction 6 months after RSD, while only thirteen non LVH patients (68%) presented this effect post procedure.
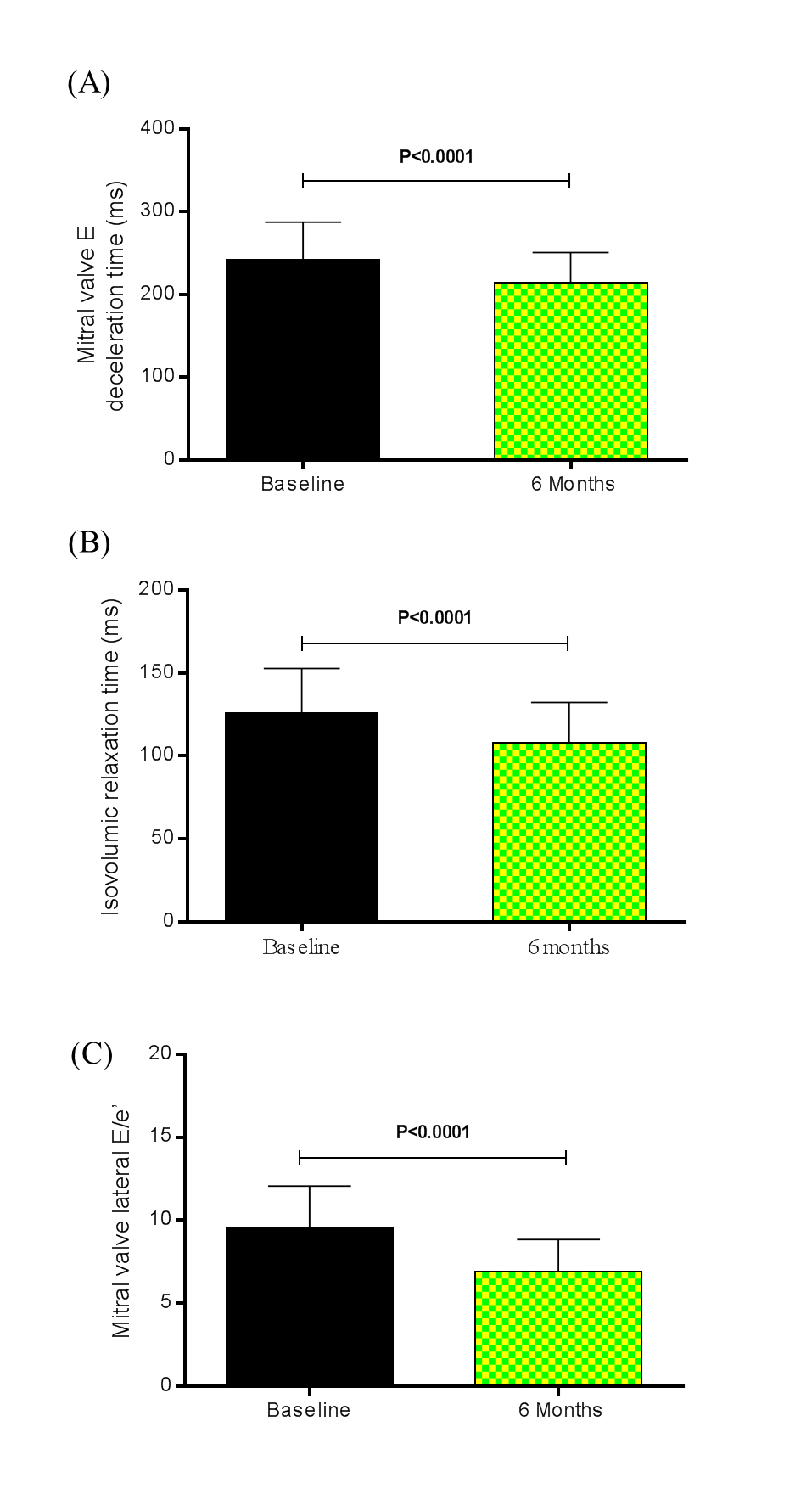
Figure 1. (A) Mitral valve E deceleration time (ms), (B) isovolumic relaxation time (ms), and (C) mitral valve lateral E/e’ at baseline and 6 months after renal sympathetic denervation (n = 45). Values are presented as mean ± SD.
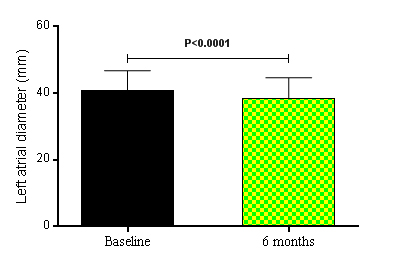
Figure 2. Left atrial diameter (mm) at baseline and 6 months after renal sympathetic denervation (n = 45). Values are presented as mean ± SD.
When the variation (∆) between baseline and the 6th month post RSD in LVH patients and non LVH patients were compared to the same parameters: mitral valve E Vmax (-6.05 ± 9.72 cm/s and -5.32 ± 7.30 cm/s, respectively, P=0.7836), mitral valve A Vmax (-10.58 ± 8.16 cm/s and -9.63 ± .10 cm/s, respectively, P=0.6874), mitral valve E/A ratio (0.06 ± 0.10 and 0.06 ± 0.14, respectively, P=0.9757), mitral valve E deceleration time (-29.65 ± 15.80 ms and -25.53 ± 20.04 ms, respectively, P=0.4439) (Figure 3A), the isovolumic relaxation time (-20.00 ± 13.78 ms and -16.05 ± 12.65 ms, respectively, P=0.3316) (Figure 3B), mitral valve lateral e’ (2.46 ± 0.95 ms and 2.00 ± 1.05 ms, respectively, P=0.1312), and mitral valve lateral E/e’ ratio (-2.82 ± 1.21 and -2.32 ± 1.27, respectively, P=0.1900) (Figure 3C) no significant difference was found. There was no difference between the ∆ LAD at baseline and at the 6th month post RSD: -2.81 ± 2.43 mm in LVH patients and -1.68 ± 2.61 mm in non LVH patients, P=0.1448.
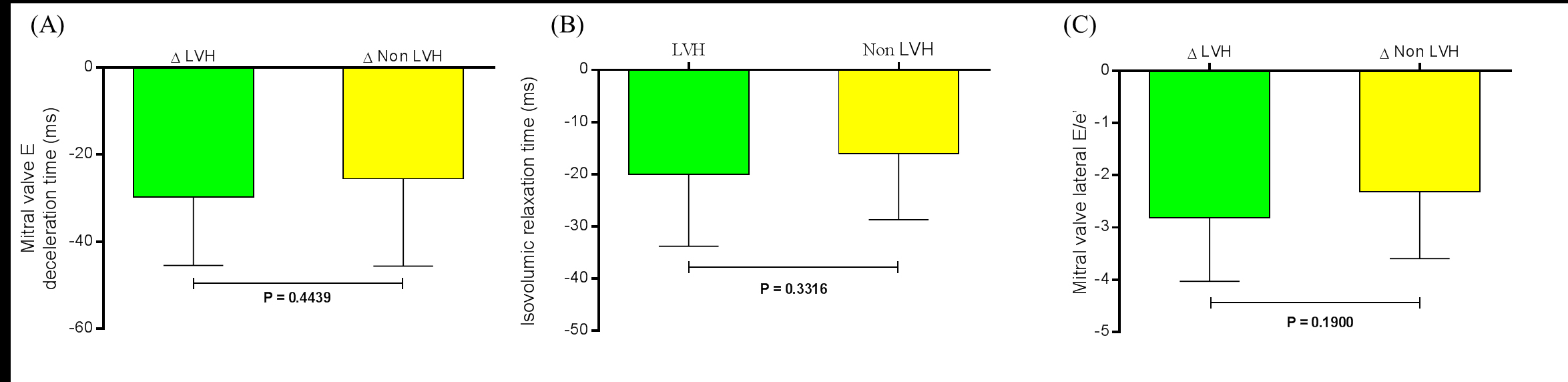
Figure 3. Variation (∆) between baseline and the 6th month post renal sympathetic denervation in LVH patients (n=26) and non LVH patients (n=19) were compared to the same parameters (A) Mitral valve E deceleration time (ms), (B) isovolumic relaxation time (ms), and (C) mitral valve lateral E/e’. Values are presented as mean±SD. LVH = left ventricular hypertrophy.
Analyzing the correlation between ∆ eGFR and ∆ mitral valve lateral E/e’ ratio we observed a positive significant value in overall patients (r=0.3151, P=0.0350), as shown in Figure 4A. However, separating groups in LVH (r=0.4037, P=0.0408,) and non LVH patients (r=0.4065, P=0.0842), only the first group presented a significant correlation (Figure 4B and 4C, respectively).
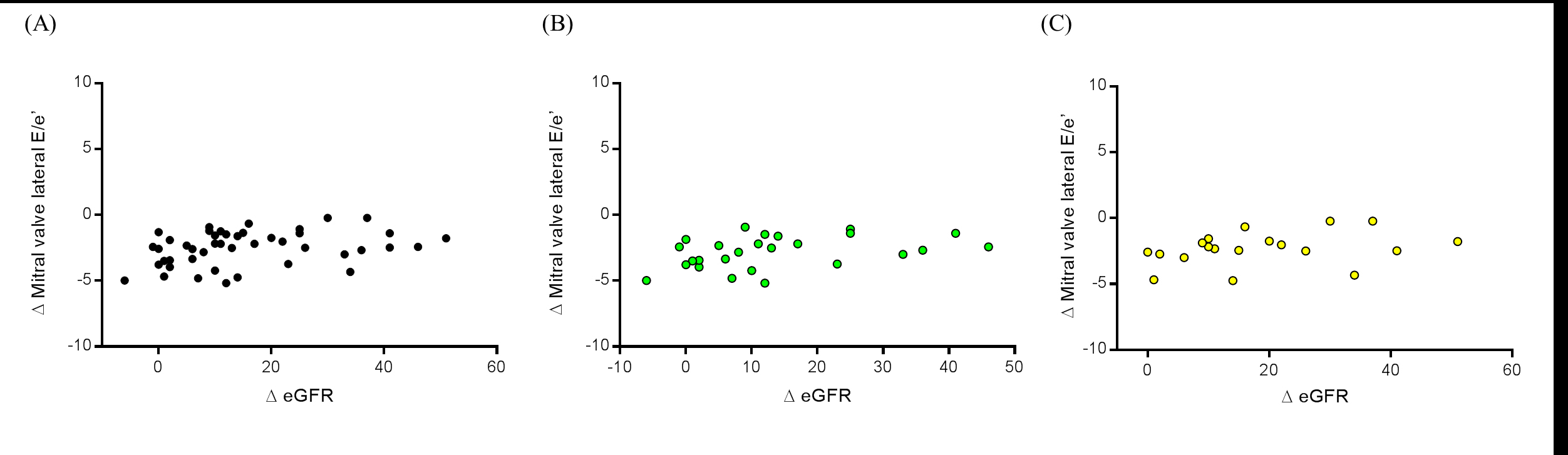
Figure 4. Correlation between the variation (Δ) of reduction mitral valve lateral E/e’ ratio at the 6th month and the variation (Δ) of change in estimated glomerular filtration rate (eGFR) in (A) all patients (n=45), (B) LVH patients (n=26), and (C) non LVH patients (n=19). LVH = left ventricular hypertrophy.
The ∆ mitral valve lateral E/e’ ratio compared among CKD stages 2 (-2.27 ± 1.36), 3 (-2.91 ± 1.11) and 4 (-2.99 ± 1.01), did not show significant different (∆ mitral valve lateral E/e’ ratio in CKD 2 vs. 3, P=0.2608; (∆ mitral valve lateral E/e’ ratio in CKD 2 vs. 4, P=0.3731; and (∆ mitral valve lateral E/e’ ratio in CKD 3 vs. 4, P=0.9885), as shown in Figure 5.
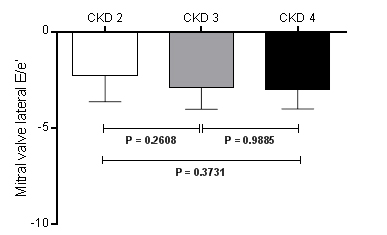
Figure 5. Variation (∆) of mitral valve lateral E/e’ ratio between baseline and the 6th month post renal sympathetic denervation in CKD stage 2 (n=22), CKD stage 3 (n=16), and CKD stage 4 (n=7). Values are presented as mean±SD. CKD = chronic kidney disease.
Safety
From the 45 patients who underwent percutaneous RSD only one patient had bleeding requiring intervention at the puncture site of the femoral artery after the end of the procedure. The complication was adequately managed by mechanical compression, fluid infusion, and blood transfusion. Real-time renal artery imaging was performed to assess eventual structural changes related to the procedure. Some small focal irregularities of the renal arteries that were present during the procedure (possibly due to minor spasm or oedema) were no longer seen postoperatively. At months 1 and 6 after the procedure all the patients underwent a new Doppler scan of renal arteries without any evidence of stenosis or flow limitation.
Chronic activation of the sympathetic nervous system is involved in the development and maintenance of arterial hypertension [27,28]. Furthermore, sympathetic over activity is a fundamental component of the signaling pathways altered in cardiac remodeling associated with hypertension [29,30].
As it is already known he control of BP reduces the rate of progression of CKD [31] and RSD is a powerful method for the control of resistant hypertension [32,33]. This procedure has also proved effective in controlling resistant hypertension even in patients with CKD. In two studies with a short follow-up period, the RSD was associated with increased eGFR [31,34,35] and the reduction of albuminuria [31,34,36]. Our data confirm the results of previous studies in relation to improving the office BP, 24-hour ABPM, number of antihypertensive drugs, creatinine values, eGFR and ACR, at the 6th month post procedure.
Several studies indicated a close relation between refractory hypertension and LVH, as well as LVH and diastolic dysfunction [37,38]. The presence of LVH is associated with an increased rate of cardiovascular events and death independent of other cardiovascular risk factors and, notably, independent of BP values [39-41]. Consistently, LVH regression was accompanied by favorable outcome [42,43]. In hypertensive patients with elevated E/e′ ratio the annual mortality rate is 10% and the ratio is considered to be a prognostic factor for the development of cardiovascular disease (CVD) [8]. Diastolic HF is an important factor that increases mortality related to the cardiovascular system in patients with CKD whose extent of kidney function deterioration differs [9].
Brandt et al. showed, in 2012, that besides the known effects on reducing blood pressure, RSD significantly reduced LV mass and improved diastolic function assessed by echocardiography, which may have important implications for prognosis in patients with resistant hypertension at high cardiovascular risk [44]. In 2014, Schirmer et al. reported that LV mass index decreased unrelated to HR at baseline (p for interaction = 0.471). The diastolic parameters E-wave deceleration time, isovolumetric relaxation time, and E’ wave velocity improved similarly in all terciles of systolic BP and heart rate (HR). Changes in LV mass and function were also unrelated to reduction in systolic BP or HR. Vascular compliance improved dependently on BP but independently of HR reduction [45]. These data corroborate our results, which show changes in LV mass index, mitral valve E deceleration time, IVRT, mitral valve E Vmax, mitral valve A Vmax, mitral valve E/A ratio, diastolic relaxation velocity of the lateral mitral annulus measured by the mitral valve lateral e’, the ratio of mitral inflow velocity to annular relaxation velocity (lateral E/e’), and LAD from baseline to 6th month post RSD. We did not observe in our patients, 6 months’ post procedure, differences between LVH and non LVH patients in all parameters aforementioned, as well as, no difference was noted in the magnitude of the change in lateral E/e’ ratio in the different CKD stages studied. Recently, our group [46] reported the improvement in some echocardiographic parameters after RSD in 15 LVH resistant hypertensive CKD patients, suggesting that RSD in this kind of patients seems to be effective in reducing LVM. We also observed a significant correlation between ∆ LVM and ∆ eGFR in overall patients. Probably this is due to the significant correlation in LVH patients, once in non LVH patients no correlation was found.
In conclusion RSD in addition to treating lesions in heart and kidneys also can treat changes in diastolic function, thereby preventing the development of diastolic heart failure. However, the LVH and the changes in aforementioned factors causing diastolic heart failure can lead to SCD in this population when it reaches the ESRD. These factors appear to be modifiable by the RSD, which would make us to think about this new tool in order to modify such factors risk, until now not modifiable. Although encouraging, our data are preliminary and need to be validated in a large population and in long term.
The relatively small sample of the study can be seen as a limitation. However, as far as we could know, the present series is the unique in the literature addressing percutaneous renal artery denervation in CKD patients and echocardiographic method. However, given the nature of the study, which is uncontrolled, our findings should be interpreted with caution. Furthermore, given the lack of other therapeutic options for resistant arterial hypertension, we are not able to compare the effects of RSD with other treatment strategies. The use of echo Doppler to assess damage in the renal arteries is in some way a limitation. However, early complications caused by the RF applications were excluded by angiography performed at the end of the procedure. Any other method, such as CT angiography, magnetic resonance angiography, or a new angiography of the renal arteries, could expose patients to additional undesirable toxic insults. Angiography using CO2 is not available in our service.
In addition, more precise methods of the assessment of GFR, such as cystatin C or iothalamate, should be used in future studies to confirm our finding regarding the effects of RSD upon the eGFR specially taking in consideration that only one measurement of serum creatinine was performed at each time point of the study.
The authors thank all the participants in this study, especially, to Pace med for the technical support.
This study was supported by the program of development of the Hospital Regional Darcy Vargas.
None declared.
- Cerasola G, Nardi E, Mulè G, Palermo A, Cusimano P, et al. (2010) Left ventricular mass in hypertensive patients with mild-to-moderate reduction of renal function, Nephrology (Carlton) 15: 203–210. [Crossref]
- Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, et al. (1999) Left ventricular mass index increase in early renal disease: impact of decline in haemoglobin. Am J Kidney Dis 34: 125–134. [Crossref]
- Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G (2005) Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 46: 320-327. [Crossref]
- Moran A, Katz R, Jenny NS, Astor B, Bluemke DA, et al. (2008) Left ventricular hypertrophy in mild and moderate reduction in kidney function determined using cardiac magnetic resonance imaging and cystatin C: the multi-ethnic study of atherosclerosis (MESA). Am J Kidney Dis 52: 839–848. [Crossref]
- Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, et al. (2006) Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int 69: 1839–1845. [Crossref]
- Hillis GS, Moller JE, Pellikka PA, Gersh BJ, Wright RS et al. (2004) Noninvasive estimation of left ventricular filling pressure by E/e’ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 43: 360-367. [Crossref]
- Maeder MT, Ammann P, Rickli H (2010) The diagnosis of heart failure with normal ejection fraction--a demanding task! Swiss Med Wkly 140: 323. [Crossref]
- Koprowski A, Gruchala M, Rynkiewicz A (2009) Management of left ventricular diastolic heart failure: is it only blood pressure control? Curr Opin Cardiol 24: 161-166. [Crossref]
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, et al. (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251-259. [Crossref]
- Grassi G (2010) Sympathetic neural activity in hypertension and related diseases. Am J Hypertens 23: 1052-1060. [Crossref]
- Grassi G. (2009) Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension 54: 690-697. [Crossref]
- Paton JF, Raizada MK (2010) Neurogenic hypertension. Exp Physiol 95: 569-571. [Crossref]
- McGrath BP, Ledingham JG, Benedict CR (1978) Catecholamines in peripheral venous plasma in patients on chronic haemodialysis. Clin Sci Mol Med 55: 89-96. [Crossref]
- Tinucci T, Abrahao SB, Santello JL, Mion D Jr. (2001) Mild chronic renal insufficiency induces sympathetic overactivity. J Hum Hypertens 15: 401-406. [Crossref]
- Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, et al. (2009) Sympathetic activation in chronic renal failure. J Am Soc Nephrol 20: 933-939. [Crossref]
- Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ (2004) Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int 65: 1568-1576. [Crossref]
- Grassi G, Bertoli S, Seravalle G (2012) Sympathetic nervous system: role in hypertension and in chronic kidney disease. Curr Opin Nephrol Hypertens 21: 46-51. [Crossref]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612. [Crossref]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, et al. (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group. J Am Soc Echocardiogr 18: 1440–1463. [Crossref]
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, et al. (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57: 450-458. [Crossref]
- Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317: 1098. [Crossref]
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, et al. (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22: 107-133. [Crossref]
- Brazilian Society of Cardiology, Brazilian Society of Hypertension, Brazilian Society of Nephrology. (2010) [VI Brazilian Guidelines on Hypertension]. Arq Bras Cardiol 95: 1–51. [Crossref]
- Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, et al. (2007) 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 28: 1462-1536. [Crossref]
- Merten GJ, Burgess WP, Rittase RA, Kennedy TP (2004) Prevention of contrast-induced nephropathy with sodium bicarbonate: an evidence-based protocol. Crit Pathw Cardiol 3: 138-143. [Crossref]
- ten Dam MA, Wetzels JF (2008) Toxicity of contrast media: an update. Neth J Med 66: 416-422. [Crossref]
- DiBona GF, Kopp UC (1997) Neural control of renal function. Physiol Rev 77: 75-197. [Crossref]
- Sobotka PA, Mahfoud F, Schlaich MP, Hoppe UC, Böhm M, et al. (2011) Sympatho-renal axis in chronic disease. Clin Res Cardiol 100: 1049-1057. [Crossref]
- Asai K, Yang GP, Geng YJ, Takagi G, Bishop S, et al. (1999) Beta-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic G(salpha) mouse. J Clin Invest 104: 551-558. [Crossref]
- Mancia G, Grassi G, Giannattasio C, Seravalle G (1999) Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 34: 724-728. [Crossref]
- Kiuchi MG, Chen S, Andrea BR, Kiuchi T, Carreira MA, et al. (2014) Renal sympathetic denervation in patients with hypertension and chronic kidney disease: does improvement in renal function follow blood pressure control? J Clin Hypertens (Greenwich) 16: 794-800. [Crossref]
- Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, et al. (2014) Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383: 622-629. [Crossref]
- Symplicity HTN-2 Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, et al. (2010) Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 376: 1903-1909. [Crossref]
- Kiuchi MG, Maia GL, de Queiroz Carreira MA, Kiuchi T, Chen S, et al. (2013) Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J 34: 2114-2121. [Crossref]
- Delacroix S. 18099 - Renal Sympathetic Denervation Increases Renal Artery Blood Flow: A Serial MRI Study in Resistant Hypertension. Data from ADELAIDE-ENLIGHTN AND RENAL BLOOD FLOW. AHA Scientific Sessions (AHA)-2014. Date: 2014/11/16.
- Ott C, Mahfoud F, Schmid A, Ditting T, Veelken R, et al. (2014) Improvement of albuminuria after renal denervation. Int J Cardiol 173: 311-315. [Crossref]
- Salles GF, Cardoso CR, Fiszman R, Muxfeldt ES. (2010) Prognostic impact of baseline and serial changes in electrocardiographic left ventricular hypertrophy in resistant hypertension. Am Heart J 159: 833-840. [Crossref]
- Douglas PS, Tallant B. (1991) Hypertrophy, fibrosis, and diastolic dysfunction in early canine experimental hypertension. J Am Coll Cardiol 17: 530-536. [croosref]
- Bombelli M, Facchetti R, Carugo S, Madotto F, Arenare F, et al. (2009) Left ventricular hypertrophy increases cardiovascular risk independently of in-office and out-of-office blood pressure values. J Hypertens 27: 2458-2464. [Crossref]
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561-1566. [Crossref]
- Ruilope LM, Schmieder RE (2008) Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 21: 500-508. [Crossref]
- Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, et al. (2004) Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA 292: 2350-2356. [Crossref]
- Pierdomenico SD, Cuccurullo F (2010) Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta-analysis. Am J Hypertens 23: 876-881. [Crossref]
- Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, et al. (2012) Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 59: 901-909. [Crossref]
- Schirmer SH, Sayed MM, Reil JC, Ukena C, Linz D, et al. (2014) Improvements in left ventricular hypertrophy and diastolic function following renal denervation: effects beyond blood pressure and heart rate reduction. J Am Coll Cardiol 63: 1916-1923. [Crossref]
- Kiuchi MG, Graciano ML, de Queiroz Carreira MA, Kiuchi T, Chen S, et al. (2016) Effects of renal sympathetic denervation in left ventricular hypertrophy in CKD refractory hypertensive patients. Int J Cardiol 202: 121-123.





