Pulmonary hemorrhage (PH), defined as extravasation of blood into airways and/or lung parenchyma, is uncommon in children. Hemoptysis, a characteristic feature of PH is rare as children tend to swallow the blood; on the other hand bleeding from upper respiratory tract and gastrointestinal bleeding (hematemesis) may mimic hemoptysis. The etiology is variable and their relative frequencies vary according to the studied population but infections, bronchiectasis, artificial airway and foreign body inhalation are the commonest. The presentation is variable depending on its origin within the respiratory tract (focal or diffuse), type of bleeding (acute or chronic/ recurrent), amount of bleeding, and presence of pre-existing lung disease. After establishing a pulmonary source of bleeding, the goals of management are to stop further bleeding and establish an etiological diagnosis. A systematic approach starting with history, clinical examination supported with laboratory tests, bronchoscopy and radiology is able to give the diagnosis in most of the cases; lung biopsy being required in only a few. Apart from the emergency measures, supportive and symptomatic management, specific therapy is dependent upon the etiology. Local causes of massive/ recurrent bleeding may be amenable to therapeutic bronchoscopy, angiography with selective embolization and surgical intervention such as resection or revascularization. In milder cases with infectious etiology, treatment of the primary disease generally suffices. Diffuse pulmonary hemorrhage associated with capillaritis requires immunosuppression and also tend to carry a poorer prognosis. Apart from the immediate complications of cardio-respiratory compromise and anemia, recurrent PH in the long term may lead to chronic respiratory insufficiency.
Hemoptysis, capillaritis, bronchoscopy, interventional radiology, immunosuppressive agents
Pulmonary hemorrhage (PH) is extravasation of blood into airways and/or lung parenchyma; whereas, hemoptysis is defined as expectoration of blood or blood tinged sputum from the lower respiratory tract [1,2]. Pulmonary haemorrhage, affecting children of all ages, may have an insidious and chronic course, or it can present as an acute life-threatening event. Though considered characteristic, hemoptysis it is a rare presentation of PH in children who tend to swallow the blood. On the other hand, blood from extra-pulmonary sources, such as those arising from the upper respiratory tract (nose, nasopharynx) or the gastrointestinal tract may be incorrectly labelled as hemoptysis. This is known as pseudo-hemoptysis. Sometimes colored food or drink may give an impression of hemoptysis, referred to as spurious Hemoptysis [3].
Pulmonary haemosiderosis is as an abnormal accumulation of haemosiderin in the lungs resulting usually from a diffuse alveolar haemorrhage. It may be primary (idiopathic) or secondary to a host of diseases [3]. Quantification of pulmonary hemorrhage is very difficult and often inaccurate in children. Though there is no consensus definition, massive hemoptysis is generally considered as bleeding exceeding 8 ml/kg of body weight in 24 hours [4]. From a clinical point of view, hemoptysis that jeopardize respiratory function and/ or leads to hemodynamic instability should be treated as “life-threatening”, a term preferred to “massive”, which requires quantification of blood lost [5].
The precise incidence of pulmonary hemorrhage in children is unknown. The incidence of Idiopathic pulmonary hemosiderosis (IPH) in Sweden during 1950-1979 was estimated to be 0.24 children/million/ year [6]. In Japan, it was estimated to be 1.23 children/million/year during 1974-1993 [7]. In the years 1993–2000, there were reports of an epidemic of an unusual form of IPH affecting 30 young infants in Cleveland, Ohio [8]. Following these reports, the US Center for Disease Control and Prevention investigated the cases and came up with a case definition for “Acute Idiopathic Pulmonary Haemorrhage” (AIPH) [9]. This was defined as an illness in a previously healthy infant (< 1 year) with a gestational age of >32 weeks, no history of neonatal medical problems that might cause pulmonary haemorrhage, and whose illness is consistent with the following criteria: (a) Abrupt or sudden onset of overt bleeding or frank evidence of blood in the airway; (b) Severe presentation leading to acute respiratory distress or respiratory failure resulting in hospitalisation in a paediatric intensive care unit with intubation and mechanical ventilation; (c) Diffuse, bilateral pulmonary infiltrates on a chest radiograph or computed tomography of the chest.
Traditionally pulmonary hemorrhage in children was classified into primary and secondary based on the presence of an identifiable systemic condition which could explain its occurrence. With the advancement in knowledge about various pathological conditions which might present as PH in children, a newer classification system have been adopted that not only encompasses its clinical presentation but its etiological spectrum and also takes into account the management considerations. Pulmonary hemorrhage is first considered on the basis of its origin, whether localized to an area in the airways/ pulmonary parenchyma or secondary to diffuse involvement of the lungs [1-4]. Table 1 presents the different causes of localized PH in children.
Table 1: Causes of localized pulmonary hemorrhage
Infection
- Bronchiectasis (cystic fibrosis, primary cilliary dyskinesia)
- Tuberculosis
- Lung abscess
- Pneumonia (bacterial, fungal, viral, parasitic, mycoplasma, legionella)
- Bronchitis
Congenital malformation
- Pulmonary sequestration
- Congenital pulmonary airway malformation (CPAM)
- Bronchogenic cyst
Bleeding diathesis
- Thrombocytopenia
- Disseminated intravascular coagulation
- Coagulopathy/ Anticoagulant therapy
- Von Willebrand Disease (VWD)
|
Trauma
- Airway laceration
- Lung contusion
- Artificial airway (tracheostomy)
- Suction catheters
- Foreign body
- Inhalation injury
Vascular anomalies
- Pulmonary thrombosis/ embolism
- Pulmonary arterio-venous (A-V) malformation
- Hemangioma
Miscellaneous
- Tumor (adenoid, carcinoid, etc)
- Lymphangioleiomyomatosis
- Tuberous sclerosis
- Pulmonary capillary hemangiomatosis
|
Conditions leading to diffuse PH are more diverse and are divided based on its pathophysiology into those having evidence of vascular inflammation and those without inflammation (Table 2).
Table 2: Causes of diffuse pulmonary hemorrhage.
Without capillaritis
Non-cardiovascular
- Idiopathic pulmonary hemosiderosis
- Heiner syndrome
- Acute idiopathic pulmonary hemorrhage of infancy (AIPH)
- Celiac Disease
- Coagulopathy
- Post-Bone marrow transplantation
- Drugs (e.g. amiodarone, propylthiouracil, penicillamine, etc.)
- Non-accidental injury/ child abuse
Cardiovascular
- Mitral stenosis
- Pulmonary veno-occlusive disease
- Pulmonary hypertension (primary or secondary)
- Chronic heart failure
|
With capillaritis
- Idiopathic pulmonary capillaritis
- Wegner’s Granulomatosis (WG)
- Systemic lupus erythematosus (SLE)
- Churg-Strauss syndrome (CSS)
- Microscopic polyangitis (MP)
- Goodpasture’s syndrome
- Polyarteritis nodosa (PAN)
- Henoch-Sconlein purpura (HSP)
- Antiphospholipid antibody (APLA) syndrome
- IgA nephropathy
- Behcet syndrome
- Cryoglobulinemia
- Drug-induced capillaritis
- Idiopathic pulmonary–renal syndrome
|
The relative frequency of different etiologies contributing to PH varies according to the patient population studied, the type of study and also the time period. Tom et al. reported 40 cases of pediatric hemoptysis during 1970-1979 from Philadelphia [10]. The commonest implicated diagnosis was pneumonia (20%), foreign body (15%) and tracheobronchitis (15%). Coss-Bu et al. in their retrospective review of cases at Texas Children’s Hospital found Cystic fibrosis (CF) (65%) to be the commonest cause of hemoptysis followed by congenital heart disease (16%) and infections other than those in CF [11]. Fabian et al. from Toronto, Canada reported 37 children with Hemoptysis and tracheobronchitis (19%) followed by tracheostomy related problems (15.5%) were the predominant causes in their cohort [12]. Batra et al. from Chicago also had similar etiological profile in their series of children with infection (28.6%) and tracheostomy related complications (14.3%) being the commonest etiologies [13]. In a more recent series (1996-2008) from Korea infectious etiology was predominant (25%) followed by CHD (17.5%) [14]. A recent systematic review of all the pediatric hemoptysis cases revealed the commonest etiology to be infective (38%) and also highlighted that in a large number no of cases was discovered (14%) [15]. Foreign body inhalation and hemorrhage related to tracheostomy use were also significant etiologies.
The lungs receive blood from two separate systems; bronchial circulation and pulmonary circulation. Bronchial Circulation is high pressure, low volume system. The vessels arise from aorta or its branches and receive about 1 % of Cardiac output. The bronchial circulation supplies conducting airways approximately down to the level of the terminal bronchioles. In contrast, pulmonary circulation is a low pressure, high capacitance circuit arising from right ventricle and supply to acinar units involved in gas exchange. Collaterals link the pulmonary and bronchial circulations naturally, but such communication can also develop secondary to diseases. The low-pressure pulmonary system tends to produce small-volume hemoptysis whereas bleeding from the bronchial system which is at systemic pressure tends to be profuse [16].
In children with bronchiectasis, chronic inflammation leads to dilated and fragile bronchial vessels which may bleed during an acute exacerbation or forceful cough. Infection (bacterial, viral, fungal) in the airways and lung parenchyma may cause local thrombosis or even necrosis leading to pulmonary hemorrhage. In congestive heart failure, alveolar hemorrhage may be secondary to mechanical injury to the endothelium as a result of high pulmonary capillary pressures [17].
In immune mediated pulmonary hemorrhagic syndromes auto-antibodies such as ANCA (Anti-Neutrophil Cytoplasmic Antibodies), AECA (Anti-Endothelial Cell Antibodies), anti-Glomerular Basement (GBM) antibodies and anti-phospholipid antibodies (APLA) lead to inflammation and damage of the vessel wall. The primary events triggering these vasculitidis are not known; several hypothesis have been proposed infectious agents can trigger and perpetuate such events [16].
Initial reports from a cluster in Cleveland suggested a possible association between AIPH and exposure to Stachybotrys chartarum (also known as Stachybotrys atra) toxin; however, subsequent analyses have disputed the proposed association [18,19].
Presence of hemosiderin laden macrophages (HLM) in the lung and airways is considered pathognomonic of pulmonary hemorrhage. They appear 3 days following an episode of hemorrhage, peak at day 7-10 with hemosiderin staining in 60% of macrophages and are still found at 2 months in 10% [20]. HLM’s confirm alveolar hemorrhage but it is not specific for any etiology and they may also be present in some interstitial lung diseases (ILD). It can be seen in sputum, gastric aspirate or bronchoalveolar lavage (BAL). An HLM index (percentage of macrophages positive for hemosiderin) of 35% was 100% sensitive and 96% specific in diagnosis of PH in pediatric population [21].
Clinically, IPH is characterized with a triad of hemoptysis, diffuse parenchymal infiltrates on chest radiographs, and iron deficiency anaemia; alveolar hemorrhage should be suspected if at least 2 of these are present [16]. In cases of haemorrhage with capillaritis, neutrophilic invasion of the interstitium followed by fibrinoid necrosis of alveolar walls can be found, with some thrombi in capillaries and venules. Alveolar epithelium may be thickened with hyperplasia of type II pneumocytes. Staining for collagen will demonstrate mild interstitial fibrosis, more prominent with chronic bleeding. Chronic cases may exhibit organizing pneumonia pattern with vascular encrustation by hemosiderin, also called as ‘endogenous pneumoconiosis’. Presence of predominantly intact RBCs in the biopsy specimen is suggestive of trauma during biopsy. Conducting airways contain large amounts of mucus in response to bleeding, and in chronic bleeding a bronchitic picture with goblet cell hyperplasia will develop [17].
The 3 characteristic histopathological patterns of diffuse alveolar hemorrhage (DAH) are: (a) DAH associated with vasculitis or capillaritis-most common pattern, closely associated with systemic vasculitis e.g. SLE; (b) ‘Bland’ alveolar hemorrhage-RBCs leak into alveoli without any evidence of inflammation or destruction of vessels e.g. IPH; (c) DAH associated with another process or condition-direct extravasation of RBCs, no evidence of vasculitis e.g. inhalation or cytotoxic drug therapy [22].
A systematic approach to the child with suspected PH is essential to: (1) diagnose the severity of pulmonary hemorrhage; (2) determine the specific etiology; (3) institute timely and appropriate therapy. The first consideration while attending to a child with suspected PH is to ascertain whether it is “life threatening” evident by cardio-respiratory compromise. In the severe acute cases, the first priority is to ensure a stable airway, breathing and circulatory status with the help of standard approach applied to any other emergency. After ensuring stabilization, the second priority is to establish the diagnosis of PH. Differentiating between hematemesis and hemoptysis can be difficult in children, especially in infants; Table 3 summarizes the clinical and laboratory features that help in the same.
Table 3: Features differentiating hemoptysis and hematemesis [1].
Hemoptysis |
Hematemesis |
Prodrome of tingling in throat or gurgling in chest |
Prodrome of nausea and abdominal discomfort |
Blood coughed out |
Blood vomited out |
Frothy, bright red in colour |
Deep purple, brown or “coffee ground” in colour |
Mixed with sputum |
May contain food particles |
Preceded by cough and followed by respiratory distress |
Usually no respiratory distress |
History of pre-existing lung disease |
Pre-existent liver or GI disease |
Associated CXR abnormality |
May be associated with jaundice |
Once it is ensured that the source of the bleeding is airways and/ or lungs, a step wise approach starting with history and clinical examination is undertaken to diagnose the etiology of PH.
- Detailed h/o presenting signs & symptoms: onset, progression, amount of bleeding, associated symptoms of fever, vomiting, lethargy, difficulty in breathing, etc.
- H/O weight loss, chest pain, chest trauma, chocking/ foreign body inhalation
- Easy fatigability, exercise intolerance
- Similar past episodes relieved in 3-4 days; blood transfusion during these episodes
- Bleeding from any other sites and prolonged/ inappropriate bleeding following trauma
- H/O pre-existing lung disease
- Other systemic illness: hematuria, growth failure, diarrhea, rash, arthritis
- Drug history
- Vitals: tachypnea, retractions, hypoxemia, tachycardia, fever, crackles, wheezing
- Eye examination (episcleritis, uveitis, or retinal vasculitis)
- Nose, nasopharynx and oral cavity for possible source of bleeding
- Skin (rash)
- Evidence of cardiovascular disease
The clinical clues that help to suspect etiological diagnosis are mentioned in table 4.
Table 4: Diagnostic clues from history and examination.
History
- Acute fever, cough, chest pain-pneumonia
- Prolonged fever, cough and weight loss-TB, malignancy
- Recurrent cough, productive sputum-bronchiectasis
- Dyspnea on exertion, fatigue, nocturnal dyspnea, frothy pink sputum-cardiac
- Immobilization, chest pain, calf pain-pulmonary embolism
- Uncontrolled asthma-allergic bronchopulmonary aspergillosis, churg strauss syndrome
- Travel to endemic regions-TB, Schistosomiasis, Paragonimiasis
|
Examination
- Pallor, clubbing, growth failure, organomegaly, hypoxemia-chronic/ recurrent bleeding
- Bleeding from other sites-bleeding diathesis
- Hypertension-collagen vascular disease
- Episcleritis, uveitis, or retinal vasculitis-vasculitidis
- Nasal septal erosion or ulcer-Wegner’s granulomatosis
- Skin rash-leukocytoclastic vasculitis
- Telangiectasia or hemangioma-pulmonary A-V malformation
- Dullness to percussion and crepitation-pneumonia
- Unequal chest wall movement and air entry, localised wheeze-endobronchial growth, FB
- Pleural rub-pneumonia, collagen vascular disease
- Murmur, loud P2-cardiac disease
|
- Complete blood count, RBC indices and peripheral smear: Severity of anemia may provide a clue to the severity of the hemorrhage. Microcytic, hypochromic anemia with marked aniso-poikilocytosis is a feature of chronic diffuse PH while acute blood loss will cause normocytic, normochromic anemia. Neutrophillic leucocytosis with a shift to left is indicative of bacterial infection, while parasitic infections are characterized by eosinophillia.
- Coagulation profile (Prothrombin time, activated partial thromboplastine time, D-dimer): Deranged coagulation profile points towards a coagulation disorder and elevated D-dimer is a marker of vascular thrombosis and possible pulmonary embolism.
- Erythrocyte sediment rate (ESR) & C-reactive protein (CRP): Elevated ESR and CRP suggests inflammation either due to infection or collagen vascular disease.
- RFT/LFT: Chronic liver disease may lead to coagulopathy and PH. A deranged RFT is seen in cases of ‘pulmonary-renal syndrome while evidence of hepatitis may be seen in cases of systemic vasculitis.
- Sputum for bacteria, fungus and mycobacter: these may provide the etiologic diagnosis in cases of suspected infectious etiology.
- Urine analysis: Proteinuria with active urinary sediments suggestive of nephritis indicates a diagnosis of pulmonary-renal syndromes (e.g. SLE, PAN, etc)
- ANCA (P-ANCA & C-ANCA), anti GBM antibodies, APLA, ANA: anti GBM antibodies for Good pasture’s syndrome, C-ANCA for Wegners granulomatosis, etc.
- Celiac serology
- ECHO: to r/o cardiac causes
- Total IgE: elevated in cases of CSS and Cow’s milk protein allergy (CMPA)
Chest x-ray (CXR)-There are no pathognomonic radiographic finding for PH. Acute bleeding generally presents as patchy or diffuse alveolar opacities, perihilar or basilar, may be migratory (Figure 1). Other findings are hilar lymphadenopathy, hyperinflation, and prominent horizontal fissure with resolution over few days. Chronic and recurrent PH is characterized by reticular-interstitial opacities. Occasionally, CXR may be normal. Important etiological clues can also be obtained from CXR such as unilateral air trapping with hyperinflation may suggest foreign body aspiration.
Computed tomography (CT)-The role of CT scan in evaluation of hemoptysis includes: (a) depiction of underlying disease including diagnosis of vascular abnormalities; (b) assessment of consequences of hemorrhage and (c) localizing site for biopsy. Acute hemorrhage presents as areas of consolidation interspersed with areas of ground-glass attenuation and preserved normal areas (figure 1); fibrosis in seen in chronic cases [23].
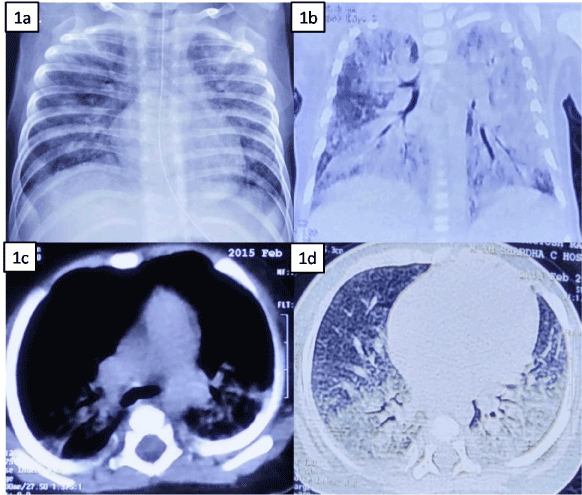
Figure 1. A-6-months old male infant with 1a-chest x-ray showing bilateral, patchy parenchymal infiltrates with normal cardiac shadow and mediastinum; 1b-Axial image of contrast enhanced computed tomography scan (CECT) of chest showing bilateral diffuse consolidation (left > right) with air-bronchogram; 1c-Coronal image (mediastinal view) of CECT chest showing no mediastinal pathology; 1d- Coronal image (parenchymal view) of CECT chest showing bilateral basal consolidation, no pleural effusion.
Bronchoscopy: Early bronchoscopy is indicated primarily to document alveolar hemorrhage, identify the site of bleeding in focal cases, to exclude infection and also to perform certain therapeutic procedures. Fibreoptic bronchoscopy allows more detailed evaluation of distal bronchial tree. However, it does not permit effective ventilation and removal of blood clots or foreign body, both of which are possible easily with rigid bronchoscopy. Batra et al. performed rigid bronchoscopy in 18 children with hemoptysis [13]. The various findings included: blood, mucosal inflammation, purulence, tracheal abrasions, granulation tissue and bronchial mass with a diagnostic yield of 61%. Various other studies reported a diagnostic yield of 40-100% for bronchoscopy in the assessment of pediatric PH [4,24].
Pulmonary function test (PFT): An obstructive pattern is observed in acute hemorrhage and restrictive changes in chronic cases. Increased diffusing capacity of carbon monoxide (DLCO) is a characteristic feature. Exhaled nitric oxide (FeNO) is decreased. The drawbacks are that these changes are not diagnostic and the child may not able to perform a PFT in acute condition.
Lung biopsy: It is resorted to in cases where the diagnosis remains elusive even after the above mentioned investigations. Biopsy of other easily accessible sites such as upper airway lesions in Wegner’s granulomatosis and renal biopsy in Good pasture's syndrome may suffice. Lung biopsy may be ‘Open’, Video-assiated thoracoscopy (VATS) guided, transbronchial or transthoracic needle core. The last 2 techniques rarely provide adequate tissue sample to arrive at a histopathological diagnosis in children with DAH. The decision between an open and thoracoscopic methods depends primary upon the availability of the facilities.
Management of the child with PH depends on two important issues – the underlying cause and the severity of the bleeding. The three goals of therapy are: to prevent asphyxiation, stop the bleeding, and treat the primary cause. Detailed discussion on the management of all the etiologies of PH in children is out of the purview of this article and discussion here focuses on the broad principles and available options.
In cases with minor PH with a discernable cause, treatment of the underlying cause is generally sufficient, e.g. antibiotics for pneumonia or acute exacerbation of bronchiectasis. Massive PH requires additional active measures to stop the bleeding. In cases with focal source of hemorrhage the various options available are: endoscopic balloon occlusion of a lobe or main bronchus, topical airway vasoconstrictors, use of Nd-YAG laser, CO2 laser bronchoscopy, endoscopic tumor excision, transcatheter embolization of bronchial vessels and lobectomy [1].
Selective embolization of bronchial arteries is technically challenging but immediate cessation of hemorrhage was could be achieved in 77% and control beyond 3 months in 45% [25]. Complications of embolization include transient fever, chest pain, back pain, dysphagia, bowel necrosis, or transverse myelitis.
Immunosuppressive agents form the mainstay of therapy in cases of DAH with capillaritis. The treatment regimen is usually divided into an early induction therapy (3-6 months), prolonged maintenance therapy (1-2 years) followed by a very slow tapering in an attempt to withdraw the medications. The induction is done with oral/ intravenous (IV) pulse corticosteroid, IV cyclophosphamide, intravenous immunoglobulin (IVIG) and plasmapharesis depending on the severity of presentation. Maintanence therapy comprises of low dose oral prednisone and methotrexate or other steroid sparing agents such as Azathioprine, Mycophenolate mofetil & Leflunamide. Biological agents (Rituximab, infliximab) are tried in refractory cases with variable success. Newer therapeutic approaches such as intrapulmonary instillation of activated recombinant factor VII (rFVIIa) has been used successfully [26].
The commonest cause of mortality in acute cases is due to asphyxiation followed by respiratory failure and hemorrhagic shock. In recurrent/ chronic cases pulmonary fibrosis leads to chronic respiratory insufficiency, frequently complicated by pulmonary hypertension and cor-pulmonale. The prognosis primarily depends on the underlying etiology and the severity of PH. In cases of IPH the clinical course is highly variable and the prognosis is overall poor. Higher age at diagnosis, history of hemoptysis or jaundice at presentation was predictive of a partial or poor response to therapy [27]. The long term survival in DAH with various etiologies is presented in table 6 [28].
Table 5: Distinguishing features of common causes of DAH [16].
|
WG |
MPA |
GS |
PC |
SLE |
IPH |
Alveolar hemorrhage |
++ |
++++ |
++++ |
++++ |
+ |
++++ |
Glomerulonephritis |
++++ |
++++ |
++++ |
- |
++++ |
- |
Elevated ESR/CRP |
++++ |
++++ |
+ |
++++ |
++++ |
+ |
Serologies |
c-ANCA |
p-ANCA |
Anti-GBM |
C- ANCA/ p-ANCA |
ANA |
- |
Extra-renal findings |
+++ |
+++ |
- |
- |
++++ |
- |
WG-Wegners granulomatosis; MPA-microscopic polyangitis; GS- Good pastures syndrome; PC-pumonary capillaritis; SLE-systemic lupus erythematosus; IPH-idiopathic pulmonary hemosiderosis
Table 6: Long term survival in children with DAH due to different etiologies.
|
WG |
MPA |
CSS |
GPS |
SLE |
IPH |
2- year survival |
35%-37% |
25 % |
20-50 % |
33-50 % |
50-90 % |
25% | 2021 Copyright OAT. All rights reserv
5-year survival |
50% |
35-40 % |
20-30 % |
80 % |
80 % |
5-15 % |
Pulmonary hemorrhage in children, though rare, can be potentially fatal and may also lead to long term morbidities. Infections continue to be a major cause of PH in children. A detailed history with a focused physical examination coupled with judicious use of laboratory and radiological investigations are able to clinch the diagnosis in most of the cases; lung biopsy being required rarely. The management depends on the type and severity of bleeding and also the underlying etiology.
- Gaude GS (2010) Hemoptysis in children. Indian Pediatr 47: 245-254. [Crossref]
- Wong TW (2012) Children with life-threatening pulmonary hemorrhage. Journal of Paediatric Respirology and Critical Care 8: 2012.
- Godfrey S (2004) Pulmonary hemorrhage/hemoptysis in children. Pediatr Pulmonol 37: 476-484. [Crossref]
- Pianosi P, al-sadoon H (1996) Hemoptysis in children. Pediatr Rev 17: 344-348. [Crossref]
- Ibrahim WH (2008) Massive haemoptysis: the definition should be revised. Eur Respir J 32: 1131-1132. [Crossref]
- Kjellman B, Elinder G, Garwicz S, Svan H (1984) Idiopathic pulmonary haemosiderosis in Swedish children. Acta Paediatr Scand 73: 584-588. [Crossref]
- Ohga S, Takahashi K, Miyazaki S, Kato H, Ueda K (1995) Idiopathic pulmonary haemosiderosis in Japan: 39 possible cases from a survey questionnaire. Eur J Pediatr 154: 994-995. [Crossref]
- Centers for Disease Control and Prevention (2014) Investigation of acute idiopathic pulmonary hemorrhage among infants in Massachusetts, December 2002–June 2003. MMWR Morb Mortal Wkly Rep. 53: 817-820. [Crossref]
- Centers for Disease Control and prevention (2004) Acute idiopathic pulmonary hemorrhage among infants: recommendations from the Working Group for Investigation and Surveillance. MMWR CDC. 53: 1-12.
- Tom LW, Weisman RA, Handler SD (1980) Hemoptysis in children. Ann Otol Rhinol Laryngol 89: 419-424. [Crossref]
- Coss-Bu JA, Sachdeva RC, Bricker JT, Harrison GM, Jefferson LS (1997) Hemoptysis: a 10-year retrospective study. Pediatrics 100: E7. [Crossref]
- Fabian MC, Smitheringale A (1996) Hemoptysis in children: the hospital for sick children experience. J Otolaryngol 25: 44-45. [Crossref]
- Batra PS, Holinger LD (2001) Etiology and management of pediatric hemoptysis. Arch Otolaryngol Head Neck Surg 127: 377-382. [Crossref]
- Sim J, Kim H, Lee H, Ahn K, Lee SI (2009) Etiology of hemoptysis in children: a single institutional series of 40 cases. Allergy Asthma Immunol Res 1: 41-44. [Crossref]
- Bannister M (2017) Paediatric haemoptysis and the otorhinolaryngologist: systematic review. Int J Pediatr Otorhinolaryngol. 92: 99-102. [Crossref]
- Vece TJ, de Guzman MM, Langston C, Fan LL (2012) Diffuse Alveolar Hemorrhage in Children. In: Chernick V, BoatTF, Wilmott RW, et al., eds. Kendig & Chernick’s Textbook of Disorder of Respiratory tract in children, 8th ed. Philadelphia: WB Saunders. 848-57.
- Avital A, Springer C, Godfrey S (2000) Pulmonary haemorrhagic syndromes in children. Paediatr Respir Rev 1: 266-273. [Crossref]
- Montaña E, Etzel RA, Allan T, Horgan TE, Dearborn DG (1997) Environmental risk factors associated with pediatric idiopathic pulmonary hemorrhage and hemosiderosis in a Cleveland community. Pediatrics. 99: E5. [Crossref]
- Centers for Disease Control and Prevention (CDC) (2000) Update: Pulmonary hemorrhage/hemosiderosis among infants--Cleveland, Ohio, 1993-1996. MMWR Morb Mortal Wkly Rep. 49: 180-184. [Crossref]
- Epstein CE, Elidemir O, Colasurdo GN, Fan LL (2001) Time course of hemosiderin production by alveolar macrophages in a murine model. Chest 120: 2013-2020. [Crossref]
- Salih ZN, Akhter A, Akhter J (2006) Specificity and sensitivity of hemosiderin-laden macrophages in routine bronchoalveolar lavage in children. Arch Pathol Lab Med 130: 1684-1686. [Crossref]
- Park MS (2013) Diffuse alveolar hemorrhage. Tuberc Respir Dis (Seoul) 74: 151-162. [Crossref]
- Singh D, Bhalla AS, Veedu PT, Arora A (2013) Imaging evaluation of hemoptysis in children. World J Clin Pediatr 2: 54-64. [Crossref]
- Ulong KS, Wang CR, Lim TY (1998) Hemoptysis in children. Chang Gung Med J 21: 57-62.
- Roebuck DJ, Barnacle AM (2008) Haemoptysis and bronchial artery embolization in children. Paediatr Respir Rev 9: 95-104. [Crossref]
- Heslet L, Nielsen JD, Levi M, Sengeløv H, Johansson PI (2006) Successful pulmonary administration of activated recombinant factor VII in diffuse alveolar hemorrhage. Crit Care. 10: R177. [Crossref]
- Kabra SK, Bhargava S, Lodha R, Satyavani A, Walia M (2007) Idiopathic pulmonary hemosiderosis: clinical profile and follow up of 26 children. Indian Pediatr. 44: 333-338. [Crossref]
- Ioachimescu OC, Stoller JK (2008) Diffuse alveolar hemorrhage: diagnosing it and finding the cause. Cleve Clin J Med 75: 258, 260, 264-265. [Crossref]

