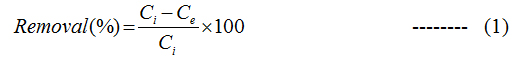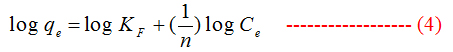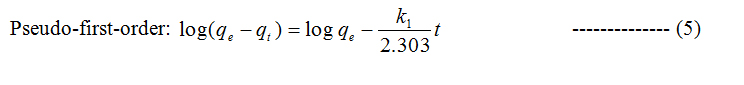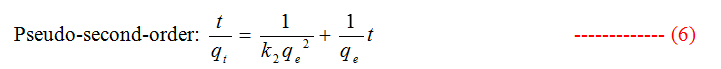Abstract
Polycyclic Aromatic Hydrocarbons (PAHs) leads to negative effects towards the environment, which have carcinogenic and mutagenic properties affecting environment as well as the aquatic system. In this study, the main aim was to investigate the adsorption and removal capacity of one of the PAHs i.e. naphthalene using synthesized Graphene and Graphene Oxide. Graphene Oxide (GO) was synthesized using rice straw and graphene (G) was synthesized using the synthesized graphene oxide. Prepared adsorbent was characterized by SEM, XRD, FTIR methods. Removal efficiency of the synthesized graphene and graphene oxide at varying conditions like adsorbent dose, temperature, pH were studied. Higher removal of naphthalene was observed at pH 7, temperature 30°C, and adsorbent dose of 2 g/L for GO and at pH 6, temperature 30°C, and adsorbent dose 2 g/L for G. Maximum percentage removal was found to be 85% and 65% for G and GO respectively. Adsorption isotherm data were analyzed by Langmuir and Freundlich models and Langmuir was found to best fit. The process was found to follow the pseudo-second order kinetics.
Key words
Graphene(G), Graphene oxide(GO), polycyclic aromatic hydrocarbons, naphthalene, isotherms, kinetics
1.0 Introduction
Now-a -days, one of the most alarming problems around the world is water pollution. This is because of the pollutants that directly or indirectly discharge into the rivers, seas consists of very harmful compounds. Pollution of river or sea may affect the living bodies present in water. Characteristics of organic pollutants depend on their size, shape, structure and functional groups presents in it and which determines its toxicity. Poly aromatic hydrocarbons (PAHs) are organic compounds containing only carbon and hydrogen, composed of multiple aromatic rings (containing delocalized π electrons) [1-3]. PAHs have fused aromatic rings, that is, rings that share one or more sides. PAHs are very widespread organic pollutants. The main source of PAH is petroleum and petrochemicals Industry. PAH pollution occurs due to a leakage of oil and it can pollute the ground water and its surrounding surface.
A simple form of PAHs is Naphthalene (C10H8). It is a white crystalline solid which has characteristic odour detectable at very low concentration. Naphthalene is a polycyclic aromatic hydrocarbon consisting of fused pair of benzene ring [4]. One of the most common disease due to expose of large amount of naphthalene is hemolytic anemia mainly in children who often ingest mothballs of naphthalene deodorants blocks. Naphthalene may damage or destroy red blood cells in human blood causing a severe anemia. The National Industrial for Occupational Safety and health has set a limit for an 8 hour time weighted average as well as a short term exposure which are 10 mg/L and 15 mg/L respectively. So naphthalene disposal through waste water should be checked before discharge.
There are a number of different techniques to remove naphthalene from waste water such as solvent extraction, membrane separation, and adsorption [5-6]. Basically, adsorption is a mass transfer technique by which a substance is transferred from the liquid phase to the surface of solid, and may bounds to the surface by physical and/or chemical interactions [7-8]. So adsorption has become one of the best alternative treatment techniques for waste water laden with the organic pollutant.
Most of the adsorbent present in the market are either expensive or have low removal efficiency. Activated carbon is among those promising adsorbent which although have high treatment efficiencies but are costly [9-12]. So researchers are engaged in search of an adsorbent which can fulfill both the conditions of high treatment efficiency and low manufacturing cost. Graphene was discovered in 2004 as an adsorbent and used as a newly emerging member of carbon materials The Graphene Oxide (GO) is a precursor of grapheme and retain much of the properties of highly valued super material of pure graphene [13-15]. It is generally obtained through a strong oxidation of graphite by modified Hummer’s method [16-21].
In this study G/GO was prepared from agricultural waste, rice straw and the removal efficiency of naphthalene using the G/GO was studied.
Materials and methods
Reagents
The reactants used in this study were naphthalene (C10H8) as the adsorbate, sodium hydroxide NaOH), hydrochloric acid (HCl) 37%, and distilled water. Rice straw was collected from local rice mill of West Bengal, India. Other reactants used for preparation of graphene oxide such as potassium permanganate (KMnO4), concentrated sulfuric acid (H2SO4, 98 wt.%), hydrochloric acid (HCl, 36 wt.%), hydrogen peroxide(H2O2, 30 wt.% ) were purchased from Merck, Germany. All chemicals were of analytical grade.
Synthesis of graphene oxide
Briefly, 10 g of rice straw was washed with distilled water and oven dried for 8 hr. The oven dried straw were sieved and carbonized in a fabricated tubular furnace reactor. The temperature of the reactor was maintained at 500C for 1 hr in under continuous nitrogen flow. The resulting solid were cooled and 100 ml of concentrated H2SO4 was added with the solid carbon materials at continuous stirring. Fixed amount of KMnO4 was slowly added in the mixture and stirring was continued for 30minutes in the ice-water bath. Then 75 ml distilled water was added to it and was placed in an incubator shaker at 45°C for 3 hours. Meanwhile 100 ml distilled water and 75 ml H2O2 aqueous solution was added in the mixture. The yellow slurry was obtained. The slurry mixture was placed in a digital ultra-sonicator for 15 minutes to get uniform particle size. Finally the slurry was filtered through a membrane filter paper and the solid material was washed repeatedly with deionized water. The synthesized GO was dried in hot air oven at 60°C for 10 hours.
Synthesis of graphene
For the synthesis of graphene, fixed amount of synthesis GO was added with distilled water at constant stirring. After that hydrazine hydrate was added to the mixture. The slurry mixture was filtered through a membrane filter paper and dried at 60°C for 6 hour.
Adsorbate preparation
Analytical grade of naphthalene (C10H8) was used for preparing the naphthalene solution for the purpose of the experiment. Predetermined amount of naphthalene dissolved in distilled water without adjusting the pH was taken and stored in a volumetric flask. The desired concentrations of working solution were obtained by diluting the stock solution successively.
Batch studies
Batch adsorption studies were performed to compare the removal of naphthalene from aqueous solution using both G and GO. In 250 ml Erlenmeyer flask, 100 ml solution of known concentration of naphthalene was taken and then a predetermined amount of adsorbent was added to it at 140 rpm in shaker. At different time interval the sample was collected and centrifuged at 10,000 rpm for 10 min. The supernatant was collected and was analyzed using UV-visible spectrophotometer (Lambda 25 Perkin elmer) and HPLC (Perkin Elmer) analyser. All the experiments were repeated thrice and average value has been taken. The percentage of naphthalene removal was calculated for each experiment by the following equation:
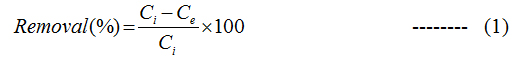
Where, Ci and Ce are the initial and final concentrations of naphthalene in the solution in mg/L. The naphthalene uptake capacity for G and GO was calculated using equation:

Results and discussions
Characterization of the adsorbent
The G and GO prepared from the rice straw were characterized using SEM (scanning electron microscopy), XRD (X-ray diffraction), FTIR ().Theanalysis was performed in order to study the morphological structure of adsorbent and to study the roughness and smoothness of the surface. The XRD analysis was carried out to investigate the intermolecular distance of the prepared sample and from the FTIR spectroscopy the functional group present on the adsorbent surface was determined. From Figure 1, 1t was observed that G has thin paper like and GO has flower like structure. The detailed analysis of characterization (SEM, XRD, FTIR) is similar to that as reported in our previous study .
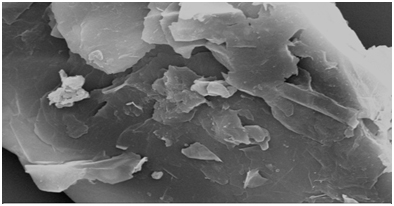
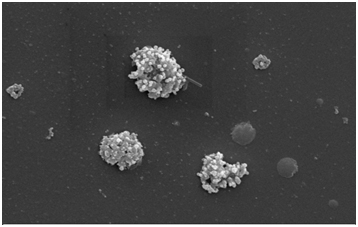
Figure 1a. SEM image of G after adsorption
Figure 1b. SEM image of GO after adsorption
Effect of adsorbent dose
The effect of adsorbent dose on the removal of naphthalene by G and GO is shown in the Figure 2. Adsorbent dose was varied over a rage of 0.05-2 g/L keeping other parameters constant. The percentage of naphthalene removal increased with an increased in adsorbent dose. It was observed that the adsorption efficiency of G was better than that of GO. The higher adsorption of naphthalene on G was mainly attributed to the π-π interaction. Compared to G, GO has lesser π-electron density and more O-containing functional groups [22]. The positive correlation between adsorbent dose and naphthalene removal can be related to an involvement of the adsorbent surface area and also an increment to the availability of more adsorption sites.
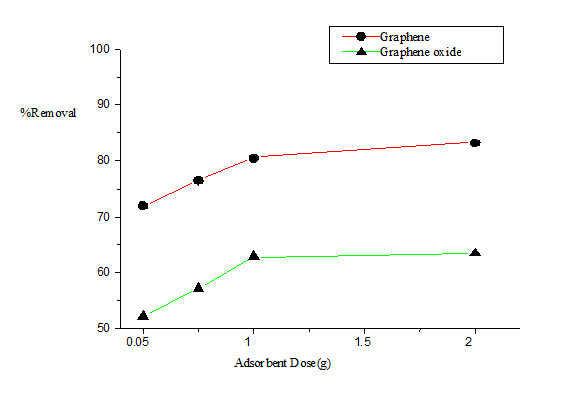
Figure 2. Plot of percentage removal of naphthalene vs. adsorbent dose.
Effect of temperature
Temperature determines whether a process is exothermic or endothermic in nature. It has been previously reported that increase in the adsorption efficiency of an adsorbent with a corresponding increase in temperature indicates that the process is endothermic in nature [15,16]. The effect of temperature on the percentage removal of naphthalene was studied in the temperature range of 298-313 K and the resultant naphthalene removal is shown in Figure 3. In this study the maximum removal was obtained at 303 K for G beyond which a decrease in percentage removal of naphthalene with a corresponding rise in temperature was noted. The same trend was observed for GO, but removal efficiency was less compared to that of G. At higher temperature weakening of the adsorptive forces between the active sites on the adsorbent and the naphthalene species may be considered as a probable explanation of the observed phenomenon.
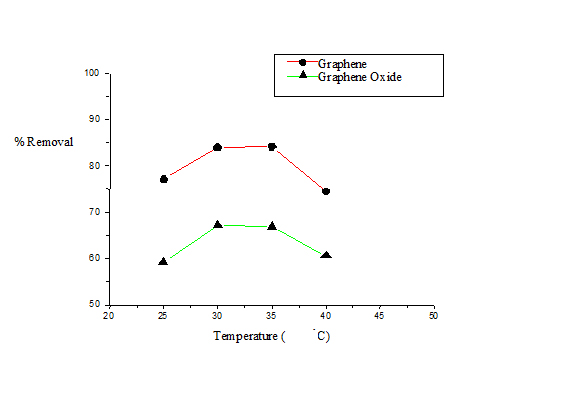
Figure 3. Plot of percentage removal of naphthalene vs. temperature for G and GO
Effect of pH
The pH of the solution has an important effect on the process of adsorption as the ionized naphthalene molecules impart electrostatic charges whose magnitude is controlled by pH of the medium. As a result the adsorption rate varies with the pH of an aqueous medium. Figure 4 shows the effect of pH on the removal efficiency of naphthalene exerted at different pH conditions ranging from 2-10. The best result was obtained at neutral pH (pH 7). In this study, G shows maximum removal efficiency of naphthalene at pH 7 (85%) whereas in case of GO the maximum removal efficiency of naphthalene to be (65%) at pH 7.
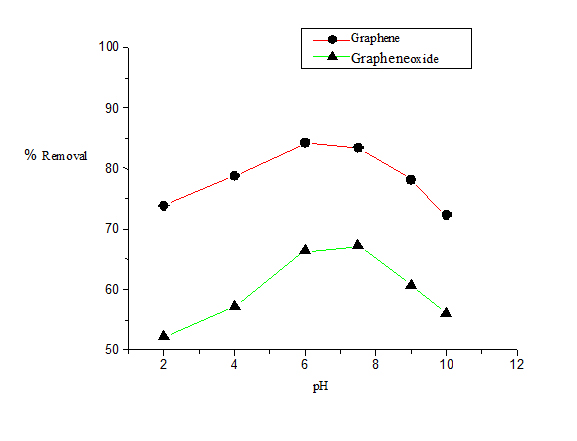
Figure 4. Plot of percentage removal of naphthalene vs. Ph
Isotherm model
An adsorption isotherm represents the equilibrium relationship between the adsorbate concentration in the liquid phase and that on the adsorbent surface at a given condition.
To describe the adsorption equilibrium data we used Langmuir and Freundlich models.
Langmuir:

Freundlich :
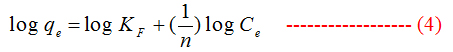
where, qe(mg g−1) and Ce(mg L−1) are the solid phase concentration and the liquid-phase concentration of naphthalene at equilibrium, respectively, qm(mg g−1) is the maximum adsorption capacity, KL(L mg−1) is the Langmuir constant, KF(mg g−1) (L mg−1), 1/n is the Freundlich constant related to sorption capacity, n is the heterogeneity factor.
For Langmuir model, Ce was plotted against Ce/qe (Figure 4). The Langmuir isotherm showed good fit to the experimental data with high correlation coefficients.
For Freundlich model, lnqe was plotted against lnCe (Figure 5) for the determination of the values of kL and qm from the slope and intercept of the plot respectively. The values of constants of Langmuir and Freundlich model are presented in Table 1 for G and Table 2 for GO.
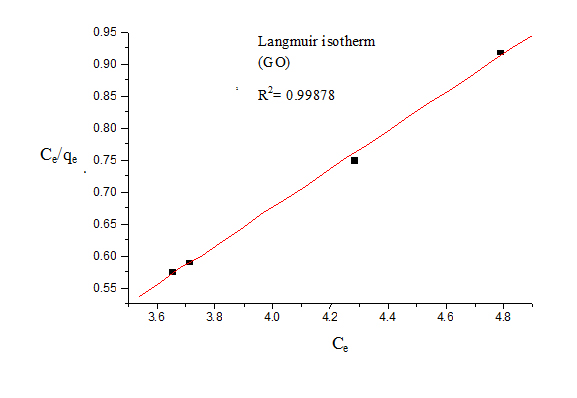
Figure 5. Langmuir isotherm (GO)
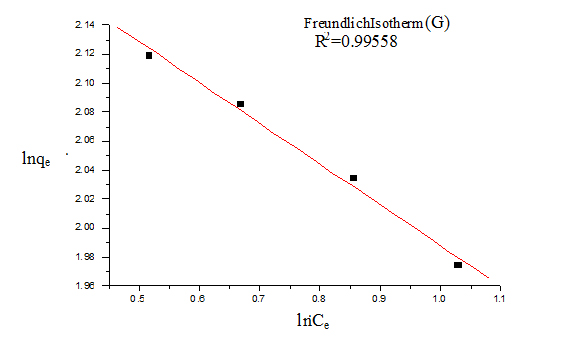
Figure 6. Freundlich isotherm (G)
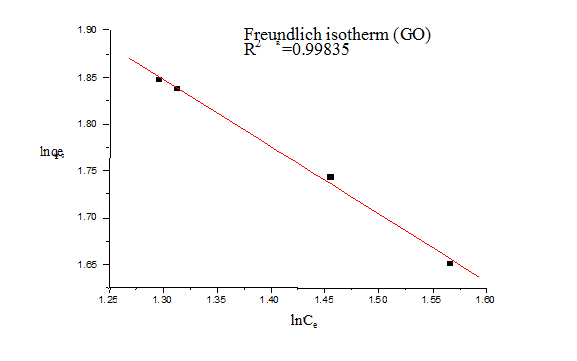
Figure 7. Freundlich isotherms (GO)
Table 1. The values of isotherm constant (G)
|
Isotherm
|
Parameter
|
Values
|
Isotherm
|
Parameter
|
Values
|
|
Langmuir
|
qm (mg g-1)
|
5.988
|
Freundlich
|
KF (mg g−1) (L mg−1)1/n
|
9.905
|
|
KL(L mg-1)
|
2.062
|
1/n
|
0.133
|
|
R2
|
0.999
|
R2
|
0.995
|
Table 2. The values of isotherm constant (GO)
|
Isotherm
|
Parameter
|
Values
|
Isotherm
|
Parameter
|
Values
|
|
Langmuir
|
qm (mg g-1)
|
3.330
|
Freundlich
|
KF (mg g−1) (L mg−1)1/n
|
16.208
|
|
|
KL(L mg-1)
|
0.571
|
|
1/n
|
0.138
|
|
|
R2
|
0.998
|
|
R2
|
0.998
|
The value of 1/n is an indicator of favourable adsorption. The values of 1/n <1 represent a favorable adsorption. In this study, the value of 1/n showed 0.133 (for G) and 0.138 (for GO), so it can be inferred that adsorption by both G and GO was favourable. The value of correlation coefficient was found to be higher for Langmuir model (R2=0.999 for G and 0.998 for GO) than Freundlich model indicating that the Langmuir adsorption model best represents the experimental values and confirming that the adsorption is monolayer.
Kinetics
To study the kinetics of adsorption study, pseudo first and second order models were considered.
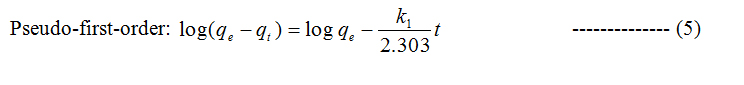
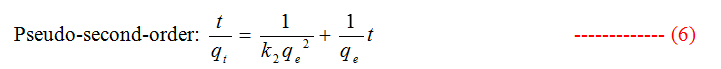
Where qt and qe are the amount of dye adsorbed at time t and at equilibrium (mg g−1), k1 (min−1) is the pseudo first-order rate constant and qt, qe are the amount of naphthalene adsorbed at time t and at equilibrium (mg g−1) respectively, k2(g mg−1 min−1) is the pseudo-second-order rate constant. t/qt values were plotted against t for G (Figure 8) and for GO (Figure 9). The correlation coefficient was found to be higher for second order for both cases. The values of the constants for the second order for G (Table 3) and for GO (Table 4) are shown in tabulated form.
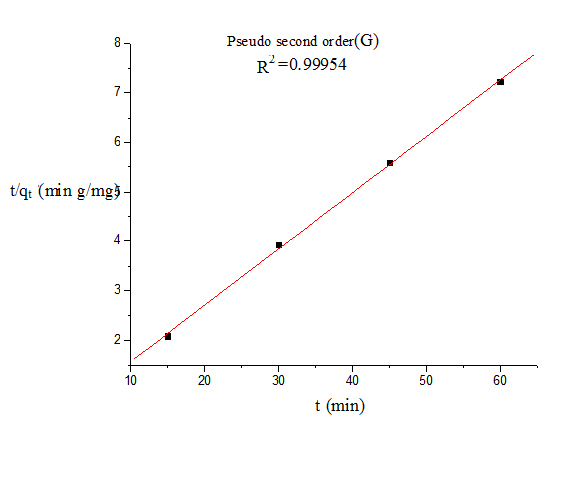
Figure 8. Pseudo second order kinetics model (G)
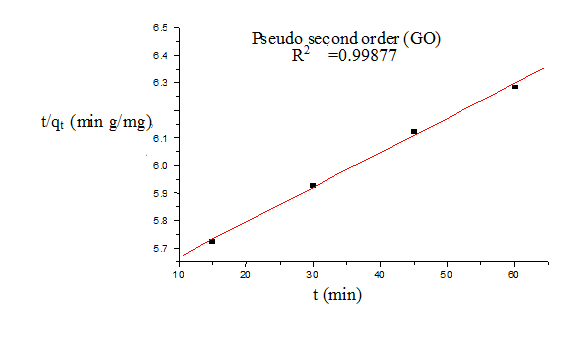
Figure 9. Pseudo second order model (GO)
Conclusion
The present findings showed that graphene and graphene oxide may be promising adsorbents for the removal of naphthalene from aqueous solutions. Characterization showed paper like structure of G and flower like structure of GO. The naphthalene was found to adsorb strongly on the surface of the adsorbents used. Batch studies showed better acceptance of graphene (with removal efficiency approximately 85%) over graphene oxide for the removal of the same concentration of naphthalene. G shows the better adsorption capacity than GO. The higher adsorption of naphthalene on G was mainly attributed to the π-π interaction. Compared to G, GO has lesser π-electron density and more O-containing functional group. Adsorption isotherms studies showed better acceptability of Langmuir model than Freundlich model which suggest that the adsorption was monolayer.
Acknowledgments
2021 Copyright OAT. All rights reserv
All authors are thankful to Ministry of Earth and Science (MOES, India) for the financial support (Research Grant-in Aid).
References
- Hu X, Li J, Chen Q, Lin Z, Yin D (2014) Combined effects of aqueous suspensions of fullerene and humic acid on the availability of polycyclic aromatic hydrocarbons: evaluated with negligible depletion solid-phase microextraction. Sci Total Environ 493: 12-21. [Crossref]
- Wenzl T, Simon R, Kleiner J, Anklam E (2006) Analytical methods for polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. Trends Anal Chem 25: 716–725
- Geetha SJA, Sanket B, Joshia J, ShaileshKathrotiyab (2013) Isolation and characterization of hydrocarbon degrading bacterial isolate from oil contaminated sites 5: 237-238.
- MansoorAnbia, SeyyedErshadMoradi (2009) Removal of naphthalene from petrochemical wastewater streams using carbon nanoporous adsorbent Applied Surface Science 255: 5041–5047.
- Papic S, Koprivanac N, Bozic AL, Metes A (2004) Removal of some reactive dyes from synthetic wastewater by combined Al(III) coagulation/carbon adsorption process. Dyes Pigm 62: 291–298.
- Zawahry MM, Kamel MM (2004) Removal of azo and anthraquinone dyes from aqueous solutions by Eichhornia Crassipes. Water Res 38: 2967-2972. [Crossref]
- Veglio F, Beolchini F (1997) “Removal of Metals by Bio-sorption: A Review,” Hydrometallurgy 3: 301-316.
- Volesky B (2000) “Detoxification of Metal Bearing Effluents: Biosorption for the Next Century,” Hydrometallurgy 59: 203-216.
- Rodri´guez-Reinoso F (1998) The role of carbon materials in heterogeneous catalysis. Carbon 36: 159–175.
- Frackowiak E, Beguin F (2001) Carbon materials for the electrochemical storage of energy in capacitors, Carbon 39: 937–950.
- Senthilkumaar S, Kalaamani P, Porkodi K, Varadarajan PR, Subburaam CV (2006) Adsorption of dissolved Reactive red dye from aqueous phase onto activated carbon prepared from agricultural waste. Bioresour Technol 97: 1618-1625. [Crossref]
- Lu W, Chung DDL (1997) Mesoporous activated carbon filaments. Carbon 35: 427–430.
- Liu T, Li Y, Du Q, Sun J, Jiao Y, et al. (2012) Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf B Biointerfaces 90: 197-203. [Crossref]
- Ai L, Zhang C, Chen Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized grapheme/magnetite composite. Journal of Hazardous Materials 192: 1515-1524.
- Ramesha GK, Kumara AV, Muralidhara HB, Sampath S (2011) Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J Colloid Interface Sci 361: 270-277. [Crossref]
- Wang C, Feng C, Gao Y, Ma X, Wu Q. Preparation of a grapheme-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chemical Engineering Journal 173: 92-97.
- Yi JW, Park J, Kim KS, Kim BH (2011) pH-responsive self-duplex of (Py)A-substituted oligodeoxyadenylate in graphene oxide solution as a molecular switch. Org Biomol Chem 9: 7434-7438. [Crossref]
- Myung S, Park J, Lee H, Kim KS, Hong S (2010) Ambipolar memory devices based on reduced graphene oxide and nanoparticles. Adv Mater 22: 2045-2049. [Crossref]
- Liang YY, Wu DQ, Feng XL (2009) Dispersion of graphene sheets in organic solvent supported by ionic interactions. Adv Mate. 21: 1679–1683.
- Su Q, Pang S, Alijani V, Li C, Feng X (2009) Composite of graphene with large aromatic molecules, Adv Mater 21: 3191–3195.
- Zhao G, Li J, Ren X, Chen C, Wang X (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45: 10454-10462. [Crossref]
- SaifulAzhar S, GhanieyLiew A, Suhardy D, Farizul K, Hafiz MD (2005) Dye removal from aqueous solution by us ing adsorption on treated sugarcane baggase. Am J Appl Sci 2: 1499-1503.
- Rathoura R, Das PB, Aikatc K (2015) Microwave-assisted synthesis of graphene and its application for adsorptive Desalination and Water Treatment 1–10 removal of malachite green: thermodynamics, kinetics and isotherm study.
- Zhiguo Pei , Lingyun Li , Lixiang Sun , Shuzhen Zhang , Xiao-quan Shan (2013) Adsorption characteristics of ,2,4-trichlorobenzene, 2,4,6-trichlorophenol, 2-naphthol and naphthalene on grapheneandgraphene oxide. Carbon 51: 156–163.