Abstract
The essence of regeneration and plasticity lies in the capacities of certain cell populations to give rise to progenies with specific functional and morphological traits. An array of molecular events directs this process (for instance, activation and de-activation of transcription or regulation of epigenetic mechanisms and controls). The unravelling of the processes that activate differentiation or de-differentiation events and the isolation and precise characterization of specific stem cell populations will open new avenues of therapy intervention in all areas of regenerative medicine, including eye pathologies. In the human anterior segment of the eye, adult stem cells can be found in the corneal limbus (the rim that separates cornea and conjunctiva). Currently, different approaches use transplantation of limbal epithelial stem cells (LESC) or corneal stromal stem cells (CSSC) to restore damaged cornea. LESC and CSSC establish a molecular dialogue that may support the maintenance of their stem phenotype. To restore corneal transparency and function other therapy approaches include the use of adult stem cells of different origins, bioengineered cells and biomaterials.
Key words
corneal limbus, LESC, mesenchymal cells, bioengineered cells
Introduction
According to The World Health Organization (WHO), corneal blindness (5.1% of total cases of blindness or visual deterioration) represents the fourth cause of blindness globally, after cataract, glaucoma and age-related macular degeneration (AMD).
Updated advances in the application of stem cells to treat diseased cornea are reviewed in this work. Also, plasticity, “stemness” and regeneration are considered in the field of therapy endeavours targeted to tackle corneal pathologies. Stem cells have an essential role in development, tissue replacement and tissue repair. They reside in niches where an orchestrated ensemble of autocrine, paracrine and endocrine factors regulate their function and fate [1-3]. Stem cells are able to proliferate and differentiate into different cell types. Hence they are very important in cell renewal, both naturally and as a therapy tool.
Difficulties in effective treatments are sometimes due to the significant extent and gravity of the lesion produced by both external insults (such as pathogenic agents or accidental damage due to burn or chemical corrosion) and genetic abnormality or ill-function. The search for new and effective treatments to restore vision is therefore a paramount. It is in this context where cell therapy may have an important niche of action.
To regenerate a tissue to its partial or complete functional state new cells with high transformation potential should be obtained. Therefore, regeneration is based on appropriate replacement. Cell can be reprogrammed to an undifferentiated state from a differentiated one [4,5]. Also, some cell populations may shift among different states of differentiation. Anuran amphibians, for example, are able to regenerate the retina by means of a transdifferentiation process of the retinal pigmented epithelium and obtain a new lens from dorsal iris pigmented epithelium [6].
Cell differentiation is an intricate route that may progress in different directions. The complexities recline in molecular “orders” that carve the final fully functional cell. But the process can, at certain points, be stopped or reversed in opposite direction, thus making the pathway more flexible and prone to required adaptations [4,7].
In general, the term “stemness” refers to the dormant state and the capacity that some cells have to differentiate in given conditions [8]. But the expression incorporates different transformation capacities (totipotent stem cells exhibit the potential to generate any cell of an organism; an embryonic stem cell, however, generates all the cells of a given organism, but the trophoblast, and the production of progenies by postnatal stem cells is restricted to the tissue where they dwell [8,9]. Common characteristics of stem cells are their ability to divide and maintain their division potential or differentiate and loose such capacity [10]. Cell division can be symmetrical, where two identical cells are generated. When cell division is asymmetrical, one daughter cell keeps “stemness” whereas the other differentiates [8,10]. How and when the cell “decides” between symmetrical and asymmetrical divisions is not fully known but both external and intrinsic factors are involved [10].
The molecular machinery (noteworthy, control and modulation of transcription) responsible for the capacity of a cell to maintain a given state of “stemness” reacts to different and numerous stimuli [11,12]. It is important therefore to define the molecular events that determine cell potencies and fates. Once we have the knowledge, and expertise, cells may be controlled and reprogrammed to suit specific needs in the ambit of therapy [7].
Corneal insults caused by different agents and diseases produce damage and alter healthy eye function. The use of stem cells, residing both in the eye and in other organs, to palliate the consequences of damage, offers promising avenues to recover visual function [13]. However, caution is always sound, and approaches should be based in solid research.
Basic architecture of the cornea
The cornea offers the appropriate molecular structure and architecture that ensures that the retina correctly receives light stimuli. Consequently, vision takes place. The molecular organization of the cornea and the absence of blood vessels maintain transparency. Three layers, namely epithelium, stroma and endothelium form the structure. The stroma contains keratocytes and is separated by two covers (Bowman and Descemet). The Bowman membrane, situated between the epithelium and the stroma and the Descemet membrane, which separates the stroma and the endothelium (a single layer of endothelial cells that extracts water from the stroma) are made of collagen [14,15]. The particular disposition of collagen fibers allows the passage of light and avoids light dispersion. Corneal transparency can be assessed by Fourier analysis [16].
When the corneal structure is damaged the pass of light may be severely impaired and vision may deteriorate or be lost. Restoration of corneal function has been approached by transplant procedures or replacement with artificial tissue [17-19]. Not always the methods are successful. It has been set forth that combined surgical and pharmacological procedures become a necessity to overcome the immunological rejection responses caused by iatrogenic intervention [20].
The cornea harbors adult stem cells
Maintenance of tissue homeostasis is an essential peculiarity in many tissues, including the cornea [21]. The XYZ hypothesis [22] establishes that the renovation of the corneal epithelium can be defined by the formulation X+Y=Z, where Z (desquamation) is the sum of X (proliferation) and Y (migration). In this proposal the X component is represented by the corneal limbus, the boundary that separates the cornea and the conjunctiva. The limbus impedes the penetration of blood vessels to the cornea form the neighbouring conjunctiva. It also feeds the cornea with metabolic products. The corneal limbus accommodates stem cells named limbal epithelial stem cells (LESC or LSCs) [23]. These cells only differentiate, in physiological conditions, to corneal epithelial cells. The pathway to a fully differentiated phenotype follows several steps (including transient amplifying cells (TAC) and post mitotic cells [24]). One relevant difficulty concerning the usefulness of limbal stem cells is their isolation as a homogeneous population. One helpful method to attain isolated LESC is the analysis of molecular markers only found in these cells. Candidates to be considered as markers have been proposed (including ABCG2 protein and cytokeratin 19 [25]). The consideration of morphological traits may also benefit proper isolation [24,26]. Besides, studies of gene expression that can be carried out by using microarrays are relevant [27,28]. Characterization of an ample group of cell surface markers, including cell adhesion molecules, cadherins, integrins or surface carbohydrates, combined with the investigation of colony forming potential and determination of transcriptional profiles are valid methodological approaches to assess LESC uniqueness [29]. In this context one consideration that has to be taken into account is that stem cells may offer changeable profiles, depending on their state (proliferation or dormant states). Therefore, the existence of many different and relevant profiles might difficult the identification and isolation of these cells [30,31]. It has been indicated that these cells are able to divide asymmetrically to ensure the pool of LESC and to provide, when needed, cells to replace the corneal epithelium [20].
The population of LESC within the limbus seems to be not homogeneous since the superior and inferior limbus accommodates more cells than the rest of the rim [31,32]. LESC harvested from the superior region of the limbus are able to produce thicker structures when cultivated [33]. The differential properties of the superior region LESC have been related to the peculiar structure of the limbal rim in this region, where crypts and projections from the stroma are abundant [15,32].
Based on evidence obtained from patients suffering from inefficient LESC, it has been proposed that the limbus is not essential for corneal epithelium turnover in physiological conditions [34]. Moreover, when the cornea is wounded, the response of LESC is not immediate but delayed some hours. Apparently, the first remedy response is carried out by the central part of the cornea [35]. Consequently, the cellular mechanisms involved in remodelling and reparation of injured or deteriorated corneal epithelium may be accomplished by separated cell populations. However, the factors and conditions that determine such processes are not fully understood.
Other adult stem cells found in the limbal corneal stroma need consideration. They are called corneal stromal stem cells (CSSC). These cells exhibit the properties of mesenchymal stem cells (clonal growth, asymmetrical divisions and ability to differentiate into multiple cell types [36]). CSSC are found in the limbalstroma, nearby Bowman´s membrane and close to limbal epithelial stem cells [36]. These cells show similar properties to bone marrow-derived mesenchymal stromal cells [37] and their identification as mesenchymal stem cells [38] can be assessed (standard criteria established by the International Society of Cellular Therapy, ISCT, see [39]). Also, they exhibit immunomodulatory properties [40,41] and their tolerogenicpotentical may be partially due to the generation of microvesicles [42]. Limbal stromal mesenchymal cells are niche cells that prevent differentiation and keep clonal growth of LESC [43,44] through mechanisms involving both soluble factors and/or microvesicles that may stimulate target cells directly or indirectly by delivering proteins and genetic material [45]. Therefore, CSSC and mesenchymal cells from other origins may have a crucial role, together with LESC, in maintaining corneal integrity. A recently described type of interstitial cells, telocytes, has been found in the corneal limbus. These cells establish direct contact (by means of telopodes) with stromal stem cells, melanocytes, macrophages and exert a paracrine influence by delivering exosomes to other cells within the limbal niche [46]. In a long-term study using a rabbit model of corneal deficiency, the reconstruction of a stem cell niche was observed [47]. Interestingly, the characterized cornea-like cells (expressing cytokeratin 12) were apparently generated from fibroblasts by EMT (epithelial-mesenchimal transition) induction. Therefore, the potential of resident corneal cells of different types should be considered and the molecular mechanisms underlying their transformation further explored.
Therapy with LESC and CSSC
LESC deficiency is a condition where limbal organization is destroyed, stem cells are not longer functional, the cornea is invaded by the conjunctiva and blood vessels penetrate corneal tissue. The consequence is that the epithelium looses architecture and thickness and is less protected against laceration [34,48,49]. The shortage of stem cells and the consequent impaired capacity to rehabilitate ruined corneal tissue produces clear symptoms, including pain, irritation and even distress and loss of vision if opacity is severe. It has to be indicated, however, that LESC deficiency is not, at present, considered as a unique and delimited condition where stem cells are no active. Often, the capacity of reaction towards injury also depends on the integrity and action of stromal cells and other cells that reside in the epithelium [50].
When an individual, due to trauma or disease, looses the capacity to regenerate its cornea with local stem cells in one eye, the healthy eye may serve as a alternative to implement a transplant [51]. Autologous transplant of cornea cultivated ex vivo was performed for the first time in patients suffering severe corneal opacity after burn [52]. To get success, in vitro cultivation of LESC demands suited requirements to obtain grown tissue in the best possible conditions for the grafting method. For instance structural material such as collagen, amniotic membranes, synthetic polymers, fibrin, silk fibroin, acellular corneal matrix, human lens capsule, etc [15,20,53-55] are needed to obtain useful tissue coats. Cloning efficiency and ROS-scavenging capacity of LESC can notably be improved by manipulating Rho-associated coiled coil kinase signalling pathways [56]. Also, the process can be improved by using other cells that feed LESC (co-cultivation with fibroblasts, for instance [57]). Mesenchymal stem cells have also feeder capacity and may transdifferenciate into epithelial-like cells when seeded on acellularxenogenic corneal matrix [54]. But cultivation procedures require further analysis aimed to improve cloning capacity of stem cells and effective tissue layers [37,58,59]. A good cultivation strategy that combines adequate bio or artificial support with feeder cells, together with the use of exogenous agents, may result in new effective applications to treat corneal wreckage [60].
Other courses of action should be taken when corneal lesion is bilateral and own resources are not available. In this case, the tactics include the use of other donors. Allograft transplant may lead to rejection. Therefore, concomitant immunosuppressant treatments must be used [61,62]. Interestingly, the application of allografts obtained by co-cultivation of LESC and CSSC or mesenchymal cells from other sources [37,54,63] may allow transplantation procedures that require no concomitant immunosuppressant treatment to palliate rejection.
Treatment aimed to cure or partially repair the harm must be based on the numerous circumstances found in the diseased eye (cause of damage, clinical situation of the patient, and so forth) [64].
Therapy with cells from other origins
Other localizations have been searched to obtain cells that can be used to treat corneal diseases. Interesting experimental findings show that corneal damage mobilizes bone marrow mesenchymal stem cells that home to the altered corneal tissue and contribute to regeneration [65]. Also, subconjunctival injection of bone marrow mesenchymal stem cells recovered corneal epithelium in an experimental acute alkali burn model 44. Bone marrow mesenchymal stem cells promote LESC survival and proliferation in a paracrine mode when co-cultured in vitro [63]. Human umbilical cord blood (hUCB) cells [66,67] or adipose-derived stem cells (ASCs) [55,68] are competent candidates to be examined in corneal repair. A recent study, using a rabbit model, adipose-derived mesenchimal cells were seeded on an acellular human cornel matrix to obtain a biocompatible graft [69]. Other locations pondered for the recruitment of suitable cells include the oral mucosa and the conjunctiva [62,70]. Also, rectal, nasal, oesophageal, anal or vaginal squamous epithelial cells should be analyzed for autologous transplant [62]. Additional therapy approaches include the application of osteo-odontokeratoprothesis (OOKP), especially when limbal transplantation is contraindicated (for instance in severe dry eye) [71,72]. Specifically, in cases of bilateral limbal stem cell deficiency, the search for autologous tissue sources is paramount [73].
Therapy with manipulated and engineered cells
Activation of certain transcription factors [74] or manipulation of gene expression [75] allows reprogramming of cells to a pluripotent state (iPS, induced pluripotent stem cell). Their broad therapeutic value is based on the capacity of iPS cells to re-differentiate into cells of the three germ layers. Paripassu, iPS cells do not raise the controversy derived from the use of human embryonic cells in research and therapy [76]. Nevertheless, the mechanisms behind reprogramming are not well understood and need attention and study. Gene expression controls and epigenetic machinery play a fundamental role in the process and should be puzzled out [76].
To be useful in therapy approaches, a programmed cell must exhibit the pertinent phenotype and respond in the adequate signalling milieu to reach the location where it is required. As an example, it has been shown that the transcription factor named Slug has a principal role in the process of migration [77]. NF-kB is also a transcription factor that influences endothelial mesenchymal transformation in the cornea in response to interleukin (IL)-1β stimulation [78]. Also, healthy maintenance of in vitro cultures is important.
The design of bioengineered tissues destined to regenerative therapies relies on in vitro models that recreate natural organogenesis. Co-cultivation of epithelial and mesenchymal feeder cells in the presence of keratinocyte growth factor and a rho kinase inhibitor permits long-term maintenance of limbal epithelial progenitor cells [79]. Amniotic membranes have been used to reconstruct ocular surfaces with variable results in part due to differences in mechanical stiffness or preparation and storage conditions [80,81]. Structural uniformity and manipulation of both physical and mechanical properties can be reached with hydrogels [82] which consist of three dimensional networks of polymers and water. Some examples are the nanofiber scaffolds, [83], type I collagen or polylactic-co-glycolic acid [84]. These materials are being assayed to secure appropriate support and environment for cell growth and stem cell profile maintenance. Reparation of corneal damage could be combined with other approaches, such as nanoparticles that may deliver drugs that facilitate wound healing and block neovascularisation [85].
Undoubtedly, knowledge of the molecular events that conduct and control differentiation and cell movement, the dialogue that different types of cells establish in their natural niches and the regulation of the main genes with a leading role in this context, will benefit clinical analysis and treatment designs [86-89]. Also, adequate animal models of limbal stem deficiency are very valuable to study interactions and to test methodologies [90].
In sum, precise characterization of both morphological traits and molecular mechanisms directing dormancy, differentiation and migration in LESC and other stem cells are of vital importance to apply them in different repair approaches [91]. It is desirable that many different options are ready for use to suit specific needs and more non-invasive approaches. Figure 1 summarizes the basic structure of the corneal limbus niche and the main corneal restoration approaches based on the use of stem cells.
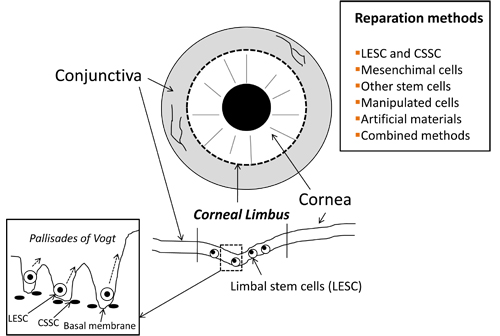
Figure 1. Basic structure of the corneal limbus niche and main therapeutical procedures used to restore corneal transparency and vision by using stem cells. LESC: Limbal Epithelial Stem Cells; CSSC: Corneal Stromal Stem Cells; TC: Telocytes.
Acknowledgements
Financial support from Grupos Consolidados Gobierno Vasco (IT437-10), Ayudas a la Investigación de Enfermedades Raras (BIOEF) and Red Patología Ocular (RETICS-RD07/0062) is gratefully acknowledged.
References
- Castro-Muñozledo F (2013) Review: corneal epithelial stem cells, their niche and wound healing. Mol Vis 19: 1600-1613. [Crossref]
- González S, Deng SX (2013) Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp Eye Res 116: 169-176. [Crossref]
- Ramasamy S, Narayanan G, Sankaran S, Yu YH, Ahmed S (2013) Neural stem cell survival factors. Arch Biochem Biophys 534: 71-87. [Crossref]
- Cobaleda C1, Busslinger M (2008) Developmental plasticity of lymphocytes. Curr Opin Immunol 20: 139-148.[Crossref]
- Kuçi S, Kuçi Z, Latifi-Pupovci H, Niethammer D, Handgretinger R, et al. (2009) Adult stem cells as an alternative source of multipotential (pluripotential) cells in regenerative medicine. Curr Stem Cell Res Ther 4: 107-117. [Crossref]
- Filoni S (2009) Retina and lens regeneration in anuran amphibians. Semin Cell DevBiol 20: 528-534. [Crossref]
- Vicente-Dueñas C, Gutiérrez de Diego J, Rodríguez FD, Jiménez R, Cobaleda C (2009) The role of cellular plasticity in cancer development. Curr Med Chem 16: 3676-3685. [Crossref]
- Verstappen J, Katsaros C, Torensma R, Von den Hoff JW (2009) A functional model for adult stem cells in epithelial tissues. Wound Repair Regen 17: 296-305. [Crossref]
- Lakshmipathy U, Verfaillie C (2005) Stem cell plasticity. Blood Rev 19: 29-38. [Crossref]
- Morrison SJ, Kimble J (2006) Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441: 1068-1074. [Crossref]
- Loebrich S, Nedivi E (2009) The function of activity-regulated genes in the nervous system. Physiol Rev 89: 1079-1103. [Crossref]
- Sivasubramaniyan K, Pal R, Totey S, Bhat VS, Totey S (2010) Rho kinase inhibitor y27632 alters the balance between pluripotency and early differentiation events in human embryonic stem cells. Curr Stem Cell Res Ther 5: 2-12.
- Jeganathan VS, Palanisamy M (2010) Treatment viability of stem cells in ophthalmology. Curr Opin Ophthalmol 21: 213-217. [Crossref]
- Obata H, Tsuru T (2007) Corneal wound healing from the perspective of keratoplasty specimens with special reference to the function of the Bowman layer and Descemet membrane. Cornea 26: S82-89. [Crossref]
- Levis H, Daniels JT (2009) New technologies in limbal epithelial stem cell transplantation. Curr Opin Biotechnol 20: 593-597. [Crossref]
- Johnsen S (2000) Transparent animals. Sci Am 282: 80-89. [Crossref]
- Melles GR, Eggink FA, Lander F, Pels E, Rietveld FJ, et al. (1998) A surgical technique for posterior lamellar keratoplasty. Cornea 17: 618-626. [Crossref]
- Melles GR (2006) Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea 25: 879-881. [Crossref]
- Sponsel WE (2009) Three cheers for DSEK (Descemet's stripping with endothelial keratoplasty)! Clin Experiment Ophthalmol 37: 831-832. [Crossref]
- McIntosh Ambrose W, Schein O, Elisseeff J (2010) A tale of two tissues: stem cells in cartilage and corneal tissue engineering. Curr Stem Cell Res Ther 5: 37-48. [Crossref]
- Chee KY, Kicic A, Wiffen SJ (2006) Limbal stem cells: the search for a marker. Clin Experiment Ophthalmol 34: 64-73. [Crossref]
- Thoft RA, Friend J (1983) The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 24: 1442-1443. [Crossref]
- Davanger M, Evensen A (1971) Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229: 560-561. [Crossref]
- Ahmad S, Kolli S, Lako M, Figueiredo F, Daniels JT (2010) Stem cell therapies for ocular surface disease. Drug Discov Today 15: 306-313. [Crossref]
- Schlötzer-Schrehardt U, Kruse FE (2005) Identification and characterization of limbal stem cells. Exp Eye Res 81: 247-264. [Crossref]
- Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, et al.(2003) Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci 44: 5125-5129. [Crossref]
- Akinci MA, Turner H, Taveras M, Wolosin JM (2009) Differential gene expression in the pig limbal side population: implications for stem cell cycling, replication, and survival. Invest Ophthalmol Vis Sci 50: 5630-5638. [Crossref]
- Bian F, Liu W, Yoon KC, Lu R, Zhou N, et al.(2010) Molecular signatures and biological pathway profiles of human corneal epithelial progenitor cells. Int J Biochem Cell Biol 42: 1142-1153. [Crossref]
- Vimalin J, Gupta N, Jambulingam M, Padmanabhan P, Madhavan HN (2012) The effect of riboflavin-UV-A treatment on corneal limbal epithelial cells--a study on human cadaver eyes. Cornea 31: 1052-1059. [Crossref]
- Brandl C, Florian C, Driemel O, Weber BH, Morsczeck C (2009) Identification of neural crest-derived stem cell-like cells from the corneal limbus of juvenile mice. Exp Eye Res 89: 209-217. [Crossref]
- Notara M, Alatza A, Gilfillan J, Harris AR, Levis HJ, et al.(2010) In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Exp Eye Res 90: 188-195. [Crossref]
- Shortt AJ, Secker GA, Notara MD, Limb GA, Khaw PT, et al. (2007) Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol 52: 483-502. [Crossref]
- Utheim TP, Raeder S, Olstad OK, Utheim OA, de La Paz M, Cheng R, et al.(2009) Comparison of the histology, gene expression profile, and phenotype of cultured human limbal epithelial cells from different limbal regions. Invest Ophthalmol Vis Sci 50: 5165-5172.
- Dua HS, Miri A, Alomar T, Yeung AM, Said DG (2009) The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology 116: 856-863. [Crossref]
- Chang CY, Green CR, McGhee CN, Sherwin T (2008) Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci 49: 5279-5286. [Crossref]
- Pinnamaneni N, Funderburgh JL (2012) Concise review: Stem cells in the corneal stroma. Stem Cells 30: 1059-1063. [Crossref]
2021 Copyright OAT. All rights reserv
- Bray LJ, George KA, Hutmacher DW, Chirila TV, Harkin DG (2012) A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials 33: 3529-3538. [Crossref]
- Branch MJ, Hashmani K, Dhillon P, Jones DR, Dua HS, et al.(2012) Mesenchymal stem cells in the human corneal limbalstroma. Invest Ophthalmol Vis Sci 53: 5109-5116. [Crossref]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al.(2006) Minimal criteria for defining multipotentmesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
- Jia Z, Jiao C, Zhao S, Li X, Ren X, et al.(2012) Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res 102: 44-49. [Crossref]
- Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, et al. (2012) Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther 20: 2143-2152. [Crossref]
- Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, et al. (2012) Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett 147: 47-54. [Crossref]
- Xie HT, Chen SY, Li GG, Tseng SC (2012) Isolation and expansion of human limbal stromal niche cells. Invest Ophthalmol Vis Sci 53: 279-286. [Crossref]
- Yao L, Li ZR, Su WR, Li YP, Lin ML, et al. (2012) Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One 7: e30842. [Crossref]
- Tetta C, Bruno S, Fonsato V, Deregibus MC, Camussi G (2011) The role of microvesicles in tissue repair. Organogenesis 7: 105-115. [Crossref]
- Luesma MJ, Gherghiceanu M, Popescu LM (2013) Telocytes and stem cells in limbus and uvea of mouse eye. J Cell Mol Med 17: 1016-1024. [Crossref]
- Kameishi S, Sugiyama H, Yamato M, Sado Y, Namiki H, et al. (2015) Remodeling of epithelial cells and basement membranes in a corneal deficiency model with long-term follow-up. Lab Invest 95: 168-179. [Crossref]
- Dua HS, Joseph A, Shanmuganathan VA, Jones RE (2003) Stem cell differentiation and the effects of deficiency. Eye (Lond) 17: 877-885. [Crossref]
- Osei-Bempong C, Figueiredo FC, Lako M (2013) Thelimbal epithelium of the eye--a review of limbal stem cell biology, disease and treatment. Bioessays 35: 211-219. [Crossref]
- Notara M, Daniels JT (2008) Biological principals and clinical potentials of limbal epithelial stem cells. Cell Tissue Res 331: 135-143. [Crossref]
- Kenyon KR, Tseng SC (1989) Limbalautograft transplantation for ocular surface disorders. Ophthalmology 96: 709-722. [Crossref]
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, et al. (1997) Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349: 990-993. [Crossref]
- MiS, Chen B, Wright B, Connon CJ (2010) Ex vivo construction of an artificial ocular surface by combination of corneal limbal epithelial cells and a compressed collagen scaffold containing keratocytes. Tissue Eng Part A16: 2091-2100.
- Zhang J, Huang C, Feng Y, Li Y, Wang W (2012) Comparison of beneficial factors for corneal wound-healing of rat mesenchymal stem cells and corneal limbal stem cells on the xenogeneic acellular corneal matrix in vitro. Mol Vis 18: 161-173. [Crossref]
- Zhang S, Espandar L, Imhof KM, Bunnell BA (2013) Differentiation of Human Adipose-derived Stem Cells along the Keratocyte Lineage In vitro. J Clin Exp Ophthalmol 4. [Crossref]
- Zhou Q1, Duan H, Wang Y, Qu M, Yang L, et al. (2013) ROCK inhibitor Y-27632 increases the cloning efficiency of limbal stem/progenitor cells by improving their adherence and ROS-scavenging capacity. Tissue Eng Part C Methods 19: 531-537. [Crossref]
- Ahmad S, Stewart R, Yung S, Kolli S, Armstrong L, et al. (2007) Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells 25: 1145-1155. [Crossref]
- Levengood SL, Murphy WL (2010) Biomaterials for high-throughput stem cell culture. Curr Stem Cell Res Ther 5: 261-267. [Crossref]
- Lin G, Xu RH (2010) Progresses and challenges in optimization of human pluripotent stem cell culture. Curr Stem Cell Res Ther 5: 207-214. [Crossref]
- De Miguel MP, Alio JL, Arnalich-Montiel F, Fuentes-Julian S, de Benito-Llopis L, et al. (2010) Cornea and ocular surface treatment. Curr Stem Cell Res Ther 5: 195-204. [Crossref]
- Tsai RJ, Tseng SC (1994) Human allograft limbal transplantation for corneal surface reconstruction. Cornea 13: 389-400. [Crossref]
- Madhira SL, Vemuganti G, Bhaduri A, Gaddipati S, Sangwan VS, et al. (2008) Culture and characterization of oral mucosal epithelial cells on human amniotic membrane for ocular surface reconstruction. Mol Vis 14: 189-196. [Crossref]
- Hu N, Zhang YY, Gu HW, Guan HJ (2012) Effects of bone marrow mesenchymal stem cells on cell proliferation and growth factor expression of limbal epithelial cells in vitro. Ophthalmic Res 48: 82-88. [Crossref]
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, et al. (2010) Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363: 147-155. [Crossref]
- Lan Y, Kodati S, Lee HS, Omoto M, Jin Y, et al. (2012) Kinetics and function of mesenchymal stem cells in corneal injury. Invest Ophthalmol Vis Sci 53: 3638-3644. [Crossref]
- Joyce NC, Harris DL, Markov V, Zhang Z, Saitta B (2012) Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol Vis 18: 547-564. [Crossref]
- Herranz AS, Gonzalo-Gobernado R, Reimers D, Asensio MJ, Rodríguez-Serrano M, et al. (2010) Applications of human umbilical cord blood cells in central nervous system regeneration. Curr Stem Cell Res Ther 5: 17-22. [Crossref]
- Bailey AM, Kapur S, Katz AJ (2010) Characterization of adipose-derived stem cells: an update. Curr Stem Cell Res Ther 5: 95-102. [Crossref]
- Alio del Barrio JL, Chiesa M, Garagorri N, Garcia-Urquia N, Fernandez-Delgado J, Bataille L, et al. (2015) Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res 132: 91-100.
- Inatomi T, Nakamura T, Koizumi N, Sotozono C, Yokoi N, et al. (2006) Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol 141: 267-275.
- Dua HS, Miri A, Said DG (2010) Contemporary limbal stem cell transplantation - a review. Clin Experiment Ophthalmol 38: 104-117. [Crossref]
- Gomaa A, Comyn O, Liu C (2010) Keratoprostheses in clinical practice - a review. Clin Experiment Ophthalmol 38: 211-224. [Crossref]
- Frank MH, Frank NY (2015) Restoring the cornea from limbal stem cells. Regen Med 10: 1-4. [Crossref]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663-676. [Crossref]
- Balasubramanian S, Babai N, Chaudhuri A, Qiu F, Bhattacharya S, et al. (2009) Non cell-autonomous reprogramming of adult ocular progenitors: generation of pluripotent stem cells without exogenous transcription factors. Stem Cells 27: 3053-3062.
- Cox JL, Rizzino A (2010) Induced pluripotent stem cells: what lies beyond the paradigm shift. ExpBiol Med (Maywood) 235: 148-158. [Crossref]
- Chandler HL, Colitz CM, Lu P, Saville WJ, Kusewitt DF (2007) The role of the slug transcription factor in cell migration during corneal re-epithelialization in the dog. Exp Eye Res 84: 400-411. [Crossref]
- Lee JG, Kay EP (2012) NF-κB is the transcription factor for FGF-2 that causes endothelial mesenchymal transformation in cornea. Invest Ophthalmol Vis Sci 53: 1530-1538. [Crossref]
- Miyashita H, Yokoo S, Yoshida S, Kawakita T, Yamagami S, et al. (2013) Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Transl Med 2: 758-765. [Crossref]
- Chen B, Jones R, Mi S, Foster J, Alcock SG, Hamley IW, et al. (2012) The mechanical properties of amniotic membrane influence its effect as a biomaterial for ocular surface repair. Soft Matter 8: 8379-8387.
- Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, et al. (2007) Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem Cells 25: 1402-1409. [Crossref]
- Wright B, Mi S, Connon CJ (2013) Towards the use of hydrogels in the treatment of limbal stem cell deficiency. Drug Discov Today 18: 79-86. [Crossref]
- Cejkova J, Trosan P, Cejka C, Lencova A, Zajicova A, et al. (2013) Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res 116: 312-323. [Crossref]
- Hirayama M, Oshima M, Tsuji T (2013) Development and prospects of organ replacement regenerative therapy. Cornea 32 Suppl 1: S13-21. [Crossref]
- Hsu CC, Peng CH, Hung KH, Lee YY, Lin TC, et al. (2015) Stem Cell Therapy for Corneal Regeneration Medicine and Contemporary Nanomedicine for Corneal Disorders. Cell Transplant 24: 1915-1930. [Crossref]
- Horswill MA, Narayan M, Warejcka DJ, Cirillo LA, Twining SS (2008) Epigenetic silencing of maspin expression occurs early in the conversion of keratocytes to fibroblasts. Exp Eye Res 86: 586-600. [Crossref]
- Katikireddy KR, Dana R, Jurkunas UV (2014) Differentiation potential of limbal fibroblasts and bone marrow mesenchymal stem cells to corneal epithelial cells. Stem Cells 32: 717-729. [Crossref]
- Karamichos D, Funderburgh ML, Hutcheon AE, Zieske JD, Du Y, et al. (2014) A role for topographic cues in the organization of collagenous matrix by corneal fibroblasts and stem cells. PLoS One 9: e86260. [Crossref]
- Li Y, Inoue T, Takamatsu F, Kobayashi T, Shiraishi A, et al. (2014) Differences between niche cells and limbal stromal cells in maintenance of corneal limbal stem cells. Invest Ophthalmol Vis Sci 55: 1453-1462. [Crossref]
- Lin Z, He H, Zhou T, Liu X, Wang Y, et al. (2013) A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest Ophthalmol Vis Sci 54: 6314-6325. [Crossref]
- Casaroli-Marano RP, Nieto-Nicolau N, Martínez-Conesa EM (2013) Progenitor cells for ocular surface regenerative therapy. Ophthalmic Res 49: 115-121. [Crossref]

