The pericardium is a watertight sac that envelops the heart and the great vessels. It is affected by various disease states, and its tough and inelastic nature means that acute accumulation of small amounts of fluid, or fibrosis and scarring processes affecting it may impede filling of cardiac chambers and reduce cardiac output. This is the main impact that the pericardium has in pathological states. In this review, we address the anatomy and physiology of the pericardium and the various pathophysiological conditions that affect it. We then review the various treatment options available to treat different pathologies affecting the pericardium.
pericardium, pericardiectomy, tamponade
The pericardium is the watertight sac that envelops the heart and the great vessels that emerge from it or drain into it. Its tough and inelastic nature implies that acute accumulation of small amounts of fluid or fibrosis and scarring of the pericardium itself impede the filling of heart chambers and reduce cardiac output, and that is the main impact that the pericardium has in pathological states. In this review, we address the anatomy and physiology of the pericardium and the various pathophysiological conditions that affect it.
The pericardium envelops the aorta and the pulmonary artery anteriorly and laterally, inferior to the innominate vein, and the superior vena cava for a few centimeters until its entry into the right atrium. It envelopes the cardiac chambers and the supradiaphragmatic inferior vena cava, and the last 1-2 centimeters of the pulmonary veins before they merge with the left atrium. The reflection of the pericardium over the pulmonary veins creates the transverse sinus superiorly and the oblique sinus inferiorly (Figure 1).
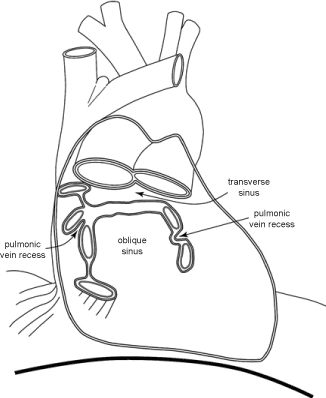
Figure 1. Shows the anatomy of the pericardium, its reflections and the location of the great vessels
The pericardium is anatomically composed of 2 layers. The inner or visceral layer is composed of a monolayer of mesothelial cells that blends with the epicardium and for all practical purposes is indistinguishable from it. The outer layer is the parietal pericardium, and this is a tough and inelastic material rich in interspersed dense collagen and elastin fibres. The parietal pericardium itself is histologically composed of 3 layers, from inside to out:
- The serosa, consisting of a surface layer of mesothelial cells and a narrow submesothelial space
- The fibrosa, containing variously oriented layers of collagen fibrils and small elastic fibers
- The epipericardial connective tissue layer, containing mainly large coarse bundles of collagen and forming part of the pericardiosternal ligament [1].
Normally about 30 milliliters of straw-coloured lymph-like fluid surround the heart within the parietal pericardium, allowing the heart to move freely within the sac.
Pericardial effusion and tamponade
Acute and chronic conditions may cause accumulation of fluid in the pericardium. These are outlined in Table 1. Acute conditions causing accumulation of fluid in the pericardium include various forms of pericarditis, both infective and inflammatory types. Chronic conditions causing a slow accumulation of fluid lead to stretching of the pericardium and delayed pressure on the cardiac chambers. Symptoms reflect the pathology causing the pericardial effusion. Viral or bacterial pericarditis causing pericardial effusions present initially with pyrexia and chest pain, followed by dyspnea on exertion, due to pressure on the left atrium and pulmonary venous congestion. Signs include an initial pericardial friction rub due to the rubbing of the pericardial surfaces together, then with accumulation of fluid, distant heart sounds, and on percussion, a large heart border, sternal dullness extending beyond the fifth costal cartilage (Dressler’s sign), and a patch of dullness at the inner part of the left lung base (Ewart’s sign) [2]. Larger effusions may produce atelectasis by compression of left lower lobe bronchus [2]. A pathognomonic sign of pericardial tamponade is pulsus paradoxus. First described by Kussmaul in 1873, this represents an exaggeration of the normal drop in systolic blood pressure, which occurs on inspiration, when negative intrathoracic pressure leads to increased venous return to the right heart. When the pericardium constricts the right heart, its free wall cannot dilate, and the ventricle can only accommodate the volume load by septal bulging to the left side, temporarily reducing left ventricular output with a consequent drop in systolic blood pressure. Pulsus paradoxus is considered present if the drop in systolic blood pressure exceeds 10 mmHg, and this can happen in a tense pericardial effusion [3]. The true explanation for pulsus paradoxus may be more complex. The enhanced pressure drop between the lowered pulmonary venous pressure on inspiration and the left atrium subjected to high intrapericardial pressures may reduce left heart filling. Downward movement of the pericardium during inspiration may contribute by further increasing intrapericardial pressure by bowstringing the pericardium [2,3].
Table 1. Causes of pericarditis
Acute |
Idiopathic |
Viral infections |
Echovirus, Coxsackie, Influenza, ebstein-barr, cytomegalovirus, Adenovirus, Varicella, Rubella, Mumps, hepatitis B&C, human-immunideficiency, Parvo B19 and Human Herpes 6 |
Bacterial infections |
purulent or septic pericarditis, tuberculosis, Coxiella burnetii, Hemophilus, Staphylococci, Chlamydia, Mycoplasma, Legionella |
Fungal infections |
Histoplasma, Aspergillosis, Blastomycosis, Candida |
Parasitary |
Echinococcus, Toxoplasma |
Systemic autoimmune diseases, eg: lupus, rheumatoid, Sjogren’s syndrome |
|
Chronic |
Idiopathic |
Bacterial infections |
tuberculosis |
Systemic autoimmune diseases |
Malignancy |
Primary eg: mesothelioma; |
secondary – lung, breast cancer, lymphoma |
Post-cardiac surgery/cardiotomy |
Post myocardial infarction |
Post-cardiac transplantation |
Post-trauma |
Metabolic |
Uremia, Myxedema |
Drug induced |
Procainamide, hydralazine, isoniazid, phenytoin, Penicillin, Doxorubicin, daunorubicin |
The size of a pericardial effusion is assessed by echocardiography and can be graded as small (echo-free space in diastole <10 mm), moderate (10–20 mm), large (>20 mm), or very large (>20 mm with compression of the heart) [4]. Progression of the pericardial effusion may result in the clinical syndrome of cardiac tamponade. This is characterized by symptoms of dizziness, dyspnea and syncope, accompanied by hypotension, an increased central venous pressure or raised jugular venous pressure, and a quiet heart, recognized as "Beck's triad" [5]. Pulsus paradoxus may be present in addition [2].
A chest radiograph reveals an enlarged and globular cardiac silhouette and echocardiogram maybe able demonstrate the presence of excess fluid in the pericardial cavity, with right atrial and right ventricular diastolic collapse in 92% and 57% of patients respectively [6]. This depends on the windows obtained for echocardiography. Following cardiac surgery, presence of some air in the pericardium and distortion of the axis of the heart may render demonstration of fluid in the pericardial cavity and its pressure on the cardiac chambers difficult to visualize and prove tamponade. However, clinical suspicion, along with echocardiographic features should help at achieving a diagnosis. High intrapericardial pressure is reflected by right atrial pressure, which is readily measurable and may aid the diagnosis, or by a raised jugular venous pressure [6]. Other echocardiographic features include inspiratory increase in isovolumetric relaxation time of around 85%, and a decrease in inspiratory early mitral flow velocity and at atrial contraction of around 43% and 25%, respectively [7]. This is accompanied by an inspiratory increase in early tricuspid flow velocities and at atrial systole of around 85% and 58% respectively [7]. Following relief of tamponade, these values drop to <25% for the right-sided velocities and <10% for the left sided velocities [7]. Furthermore, inspiratory left ventricular ejection time and aortic flow velocities can drop by 21-26%, while inspiratory pulmonary flow velocities increased 40%; all these decreased to <5-10% after relief of tamponade [7]. Echogenic pericardial fat or pulmonary shadows may give the appearances of intrapericardial clot adherent to the cardiac chambers. These can lead to a false diagnosis of tamponade and may require computerized tomogram to confirm or exclude the diagnosis of a pericardial effusion [8].
If tamponade is suspected treatment should be immediate, to prevent deterioration and development of clinical shock syndrome. This maybe initially treated by subxiphoid pericardiocentesis under echocardiographic guidance [8]. If fluid re-accumulates, then a more permanent solution may be the creation of a pericardial window to drain any future fluid build-up into the pleural space (by balloon echo-guided percutaneous balloon, or through minithoracotomy or video-assisted thoracoscopic procedures) [9,10]. An alternative is subxiphoid pericardiotomy and pericardial-peritoneal window. If tamponade is due to blood with clots, sternotomy may be needed [2]. All have an 80%-90% efficacy in relieving pericardial effusion and help diagnosis by permitting pericardial biopsy [8].
Pericardial constriction
Presentation is insidious, with fatigue, dyspnea on exertion, orthopnea, weakness, abdominal distension, and peripheral edema [11]. Signs of right-sided failure predominate, with ascites, hepatomegaly and distended veins; ankle and peripheral edema appear late, while signs of left-sided failure such as orthopnea and inspiratory crackles are less frequent [2]. Kussmaul’s sign (increased inspiratory venous distension) may be present, along with hepatojugular reflux and other evidence of and increased central venous pressure while pulsus paradoxus is infrequent [2,12]. There may be atrial fibrillation with a rapid ventricular rate, a ventricular third heart sound – ‘pericardial knock’ - due to abrupt cessation of ventricular filling in early diastole [2]. This usually occurs 0.06-0.12 seconds after the second heart sound and coincides with peak diastolic filling [13].
Dissociation of intrathoracic and intracardiac pressures with respiration
Normally there is an inspiratory decrease in all cardiac and pulmonary pressures due to the negative intrathoracic pressures. In constriction, the cardiac chambers are isolated from intrathoracic pressure changes due to the thickened pericardium. The predominant pathophysiology is the limitation of cardiac filling, not throughout the cardiac cycle as in tamponade, but towards the end of diastole due to the stiff, non-compliant fixed pericardium. Thus, early diastolic filling pressures are low and late diastolic filling pressures are much higher. This early diastolic dip and a high diastolic plateau in the right ventricle is described as the "square-root" sign (Figure 2) seen on pressure tracings obtained by during cardiac catheterization, and is suggestive of constrictive pericarditis (CP) [2,14]. Right atrial pressure tracings reveal a deep ‘y’ descent, which correlates to the nadir of the square-root sign (Figure 2) [14]. Normally, inspiration results in up to 10 mmHg drop in right atrial pressure. In constriction, the thickened pericardium prevents the right atrium from accepting inspiratory acceleration of blood from the systemic veins. Therefore, the neck veins become prominently distended during inspiration, producing Kussmaul’s sign [14]. In CP, 75% of diastolic filling essentially occurs in the first quarter of diastole, and these hearts are dependent on tachycardia to maintain cardiac output [13].
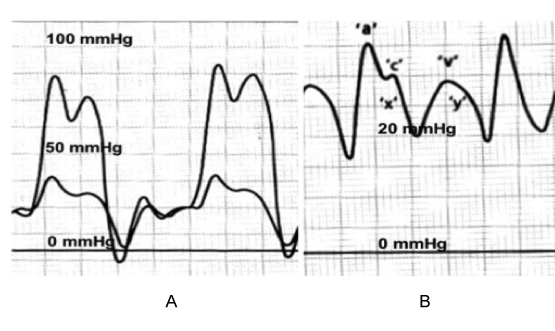
Figure 2. A. Right and left ventricular pressure tracings showing the abrupt cessation of diastolic filling with a plateau of the pressures (square root sign). B. Right and left atrial pressure tracing showing the deep ‘y’ descent.
In a dog model of pericardial constriction, Santamore and associates elegantly demonstrated that ventricles are pathologically “coupled”, so that filling of one ventricle causes a shift of the septum into the other, and the end-diastolic pressures of the right and left ventricles tend to equilibrate [15]. The total amount of blood entering the heart changes little during the respiratory cycle. In inspiration, the negative intrathoracic pressures draw volume into the great veins and thus into the right atrium and ventricle, the septum moves leftward (septal bounce) and impedes diastolic filling of the left ventricle due to the rigid pericardium. This results in 2 phenomena, a sharp rise in diastolic filling pressures in the right ventricle – square root sign in the right ventricular trace on cardiac catheterization (Figure 2), and pulsus paradoxus, due to a reduction in left ventricular output during inspiration manifested by >10 mmHg reduction in inspiratory systolic pressures. The opposite physiologic effects on septal shift and filling gradients of the ventricles are seen with expiration. As the intrathoracic pressure rises with expiration, the flow from pulmonary veins into the left ventricle increases with expiration and the septum shifts rightward, resulting in an expiratory reduction of flow velocity in the venae cavae, increased hepatic venous diastolic flow reversal, and decreased tricuspid flow velocity [13].
The main differential diagnosis for CP is Restrictive Cardiomyopathy (RCM), characterized by infiltrative processes in the myocardium, causing myocardial restriction and non-compliance. Diseases causing RCM include amyloidosis, sarcoidosis, radiation, carcinoid and anthracycline toxicity. Some of the clinical findings mimic pericardial constriction and echocardiography, CT scanning and cardiac MR may help to distinguish between the two. Distinctive myocardial speckling may be found on echo with amyloidosis or other infiltrative conditions [14]. The presence of a thickened pericardium >4 mm on Cardiac Magnetic Resonance Imaging (CMR) supports a diagnosis of CP [16]. A normal endomyocardial biopsy helps to exclude RCM, but presence of non-specific inflammation or fibrosis may cloud the issue, and the 2 conditions may co-exist if the etiology is mediastinal irradiation [17]. In the past, tuberculosis was the predominant cause of CP, but has been replaced currently by previous cardiac surgery and radiotherapy to the mediastinum [18]. Distinction between the two pathologies is important, as CP remains a surgical disease associated with good prognosis, while RCM has no surgical options and a poor prognosis.
The “classic” hemodynamic criteria used for the diagnosis of constrictive pericarditis are near-equalization of end-diastolic pressure (left ventricular end-diastolic pressure − right ventricular end-diastolic pressure (RVEDP) difference ≤5 mm Hg), pulmonary artery systolic pressure <55 mm Hg; RVEDP/ right ventricular systolic pressure (RVSP) >1/3; dip-and-plateau filling, as reflected in the height of the left ventricular rapid filling wave (RFW) (Figure 2); and lack of respiratory variation in the mean right atrial pressure [18].
This ventricular interdependence and dissociation of intrathoracic and intracardiac pressures maybe assessed with Doppler echocardiography, manifested by an inspiratory increase in peak tricuspid flow velocity and a simultaneous decrease in mitral flow velocity, with opposite changes occurring in expiration [19]. Respiratory variation of the mitral inflow peak early velocity of ≥10% predicts constrictive pericarditis with 84% sensitivity and 91% specificity and variation in the pulmonary venous peak diastolic flow velocity of ≥18% distinguished constriction with 79% sensitivity and 91% specificity [20]. Doppler tissue imaging may also be helpful in distinguishing CP from RCM. The ventricular wall motion velocities are deranged in myocardial disease but preserved in constrictive pericarditis. Since isovolumic relaxation precedes the onset of filling, alterations in its rate are less likely to be influenced by preload. A peak early velocity of longitudinal expansion of ≥8.0 cm/s differentiated constriction from restriction with 89% sensitivity and 100% specificity. A slope of ≥100 cm/s for the first aliasing contour in color M-mode flow propagation predicted patients with constriction with 74% sensitivity and 91% specificity. This technique thus complements Doppler assessment of respiratory variation in distinguishing between CP and RCM [20]. CMR is helpful in identifying features of gadolinium enhancement of pericardium, septal bounce and distended inferior vena cava [21].
However, with the onset of atrial fibrillation, the non-invasive assessments become unreliable [18]. Therefore, data obtained at cardiac catheterization remain invaluable in diagnosing CP. These assume further importance as the aetiology of CP has changed in the Western world, from TB to mediastinal radiotherapy and previous cardiac surgery, both of may cause patchy changes of inflammation and thickening of the pericardium, along with inflammation and fibrosis in the myocardium, making the diagnosis even more difficult [18]. The data obtained at cardiac catheterization include:
- 1. Elevation of mean atrial pressures >10 mm Hg
- 2. Prominent y descent in right atrial pressure tracing [22] in 94% cases – Friedreich’s sign. This is due to rapid diastolic emptying of the high-pressure right atrium.
- 3. Elevated right ventricular end-diastolic pressure > one-third of right ventricular systolic pressure. This criterion has a sensitivity of 93% and a specificity of 57% for constrictive pericarditis [13,14,18].
- 4. Square-root sign on right ventricular pressure tracing
- 5. Near-equalisation of pressures. Left and right ventricular end-diastolic pressures are within 5 mm Hg of each other [13]. The sensitivity and specificity of of this finding for the diagnosis of constrictive pericarditis were 60% and 71%, respectively [18]
- 6. Dissociation of pulmonary capillary wedge pressure from LVEDP: A difference of 5 mm Hg in the gradient between PCWP and LVEDP inspiration and expiration was 93% sensitive and 81% specific for constrictive pericarditis [18].
- 7. Increased ventricular interdependence assessed by comparing LVSP and RVSP during respiration: In patients with CP, LV and RV pressures have a reciprocal relationship throughout the respiratory cycle, whereas patients with heart failure have concordant changes in their pressures. This ventricular interdependence is 100% sensitive and 95% specific for the diagnosis of CP [18].
Criteria for the diagnosis of restrictive cardiomyopathy include [14]:
- 1. Increased jugular venous pressure
- 2. Prominent x and y descents
- 3. A small or normal-sized heart
- 4. Pulmonary congestion
- 5. Hepatic congestion
- 6. Absence of ventricular hypertrophy or dilatation
- 7. Normal to mildly depressed ventricular systolic function
In a further attempt to distinguish between CP and RCM, Aroney and coworkers performed radionuclide angiography on patients with CP and RCM and compared them with normal volunteers. There was reduction in left ventricular ejection fraction in both groups compared to healthy volunteers. They found that diastolic filling occurred more rapidly in patients with CP compared with RCM patients and normal subjects in the beginning 20-40% of diastole and reached a plateau at 50% of diastolic filling time compared to plateau at 75% of diastolic filling time in healthy volunteers [17]. They also found that time to peak diastolic filling was significantly shorter in CP than RCM and healthy volunteers [17]. This is significantly different from tamponade, where filling is restricted throughout diastole [13].
Acute pericarditis
These are outlined in Table 1. Commonest causes are idiopathic or viral etiology; specific causes include tuberculosis, neoplastic and pericarditis associated with systemic autoimmune diseases, all of which have 5% incidence of pericardial involvement, and must be actively excluded in every case [23]. Iatrogenic causes include catheter-based procedures included percutaneous coronary interventions, ablation procedures and pacemaker insertions, all of which may either excite a pericardial reaction or may result in a traumatic haemorrhagic pericarditis [24]. Features include new onset sharp central chest pain worsened by deep inspiration, pyrexia, malaise, pericardial friction rub, widespread ST elevation and PR depression on all ECG leads, and possible pericardial effusion. Investigations include blood cell count which may show leukocytosis, erythrocyte sedimentation rate and C-reactive protein levels which maybe elevated, plain chest roentgenogram which may show cardiomegaly suggestive of pericardial effusion, electrocardiogram which may show the above mentioned findings, and an echocardiogram which could confirm the presence of a pericardial effusion. Pericardiocentesis or pericardial biopsy performed percutaneously or by video-assisted thoracoscopic techniques maybe required if specific diagnoses such as tuberculosis or neoplasms are suspected [24]. Pyrexia >38°C, subacute onset, presence of a large pericardial effusion causing cardiac tamponade and lack of response to aspirin or NSAIDs after at least 1 week of therapy, myopericarditis, immunosuppression, history of trauma and oral anticoagulant therapy, predicted lack of response to conventional treatment and increased risk of complications, and were present in 15% of patients [23,25]. The remaining 85% of patients could be treated conservatively in the community with high-dose aspirin for 7-10 days with no risk of tamponade [4,25]. In the presence of a large pericardial effusion with features of tamponade, pericardiocentesis and drainage must be performed and fluid sent for cytology to exclude malignancy, and specific Zeihl-Neilsen stain and tuberculous bacilli culture or polymerase chain reaction to exclude tuberculosis, the chances of tuberculosis or malignancy being higher in these cases [26]. Presence of myopericarditis is suggested by ECG findings showing myocardial involvement in addition to pericarditis, and elevation of CKMB, and requires admission to hospital, restriction of activity for 4-6 weeks and treatment for heart failure with beta-blockade and ACE inhibitor therapy if echocardiogram suggests decreased LV function [27].
Relapsing pericarditis is of two types – incessant type, which recurs immediately following cessation of NSAID or steroid therapy, and the intermittent type, which recurs following a symptom-free gap of at least 6 weeks with no treatment. Coxsackie B viral pericarditis and autoimmune pericarditis are prone to relapses, and the latter may evolve into CP [28]. Symptoms are usually maximal in the “index” or initial attack and recurrences tend to be milder. Tamponade is rare during recurrences. Prognosis is excellent once the autoimmune diseases have been excluded. Treatment options include NSAIDs initially, colchicine for recurrences, and steroids, azathioprine and cyclophosphamide as a last resort [4,28]. Development of chronic, symptomatic, large pericardial effusions require percutaneous catheter based methods [9] or creation of a pericardial window either into the abdominal or pleural cavities for drainage [10,29].
Purulent pericarditis
This is a subtype of pericarditis occurring in the immunocompromised host [30]. Pneumonia, cancer, recent cardiac or thoracic surgery, dialysis and malignancy are predisposing factors. It is associated with mortality of 20-30%. Early complications include tamponade and septic shock, while late complications include persistent pericarditis, pericardial fibrosis and CP. Persistent pericarditis is characterized by multi-loculated pericardial purulent collections despite adequate antibiotic therapy [30]. Intrapericardial fibrinolysis maybe attempted [31] with a rationale similar to that for empyema where it has been shown to reduce the need for surgical drainage [32]. A variety of fibrinolytic agents and dosages have been suggested [30,33]. Failure of 3 attempts at fibrinolysis warrant surgical drainage, either through a sub-xiphoid approach or subtotal pericardiectomy [4,34,35] to clear the infection completely, in addition to broad-spectrum anitbiotics, and this may prevent the future development of CP in these patients.
Constrictive pericarditis
First described more than 300 years ago, constrictive pericarditis (concretio cordis) (CP) is a post-inflammatory disorder characterized by a variably thickened, fibrotic, and frequently calcified pericardium [13]. The leading cause still remains tuberculosis in the developing world, accounting for 38-83% of cases [13]. In the developed world, etiology is frequently unknown, and some cases are associated with previous pericarditis, cardiac surgery, even cardiac transplantation [36] and radiotherapy. Other rarer causes include myocardial infarctions and connective tissue disorders [37].
2021 Copyright OAT. All rights reserv
The clinical features, investigations and differential diagnoses are discussed above. Surgery remains the mainstay of treatment for CP. Pericardiectomy is performed through a median sternotomy, and is attempted without the use of heparinization or cardiopulmonary bypass unless there are other cardiac lesions that require to be addressed simultaneously using CPB. Subtotal pericardiectomy, in which the phrenic nerves limit the posterior extent of the pericardial resection, remains the procedure of choice [37,38]. A cleavage plane between myocardium and pericardium is identified and the epicardial as well as the pericardial layer is resected thereby liberating the myocardium and allowing the cardiac chambers to expand to their normal capacity. There is a theoretical benefit of decorticating the left side of the heart to avoid the theoretical risk of pulmonary edema due to initial decortication of the right heart. The pericardium is resected from the left to the right phrenic nerves. Pericardium is usually not removed from the posterior side of the left atrium and around the pulmonary veins. In the absence of a cleavage plane between the pericardium and the myocardium pericardial “meshing” has been described [37]. All constricting layers down to the myocardium are meshed by multiple cross incisions using the tip of a No. 11 surgical blade. Complications of surgery include bleeding, mediastinitis, respiratory failure, low cardiac output and a mortality of 2-6% [37,38].
Pericardial neoplasms
Primary pericardial malignancies are rare. Mesothelioma, is the commonest one, usually unresectable and almost always incurable [39]. Secondary tumors involve the pericardium by local extension or hematogenous spread and include carcinomas of the lung, esophagus, liver, stomach, breast and pancreas, lymphomas, leukemias, and thymomas, commonest being carcinomas of the lung and esophagus, and lymphoma. The pericardium, including the epicardium, may be involved by secondary tumors, followed by myocardium and endocardium [39,40]. Primary cardiac tumours spreading to the pericardium are extremely rare and include myxomas, rhabdomyomas, hemangiomas, and lipomas [40].
Carcinoma of the lung and oesophagus with limited local extension may be amenable to complete curative resection with the primary malignancy [39]. However, most primary and secondary malignancies are not amenable to surgical resection [39]. These patients often present with a symptomatic pericardial effusion, which may cause tamponade. Subxiphoid pericardiostomy and drainage is a safe procedure that provides effective and durable symptomatic relief [39]. These effusions maybe amenable to percutaneous balloon pericardial window into the pleural space to alleviate the tamponade [9]. Thoracoscopic pericardiectomy may also be performed effectively and safely in these patients, with better visualization and resection of the pericardium than percutaneous or subxiphoid techniques, and less wound related pain than an open thoracotomy [10].
Pericardial cysts
Pericardial cysts are rare, and occur predominantly in the right cardiophrenic angle. Their diagnosis is suspected after an abnormal chest X ray is obtained. Echocardiography is valuable in diagnosing the presence of a pericardial cyst. Although most pericardial cysts are asymptomatic, patients may present with chest pain and dyspnea [4]. CT and CMR may be needed to establish diagnosis. Treatment is by open or video-assisted thoracoscopic surgical resection [4,42].
The pericardium could be affected by a variety of pathologies – primary and secondary to other systemic disease. The clinical presentation critically depends on the mode of presentation – acute or chronic compression of the heart and great vessels, leading to varying degrees of reduction of cardiac output and compensatory features of right sided and/or left sided congestion. Treatment modalities are essentially aimed to relieve the compression on the heart and great vessels, and therefore to allow adequate cardiac output and reduction of right sided and/or left sided congestion.
- 1. Ishihara T, Ferrans VJ, Jones M, Boyce SW, Kawanami O, et al. (1980) Histologic and ultrastructural features of normal human parietal pericardium. Am J Cardiol 46: 744-753. [Crossref]
-
2. Baue AE, Blakemore WS (1972) The pericardium. Ann Thorac Surg 14: 81-106. [Crossref]
-
3. McGregor M (1979) Current concepts: pulsus paradoxus. N Engl J Med 301: 480-482. [Crossref]
-
4. Maisch B, Seferovic PM, Ristic AD, Erbel R, Rienmuller R, et al. (2004) Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J 25: 587-610. [Crossref]
-
5. Sternbach G, Claude Beck (1988) cardiac compression triads. J Emerg Med 6: 417-419. [Crossref]
-
6. Levine MJ, Lorell BH, Diver DJ, Come PC (1991) Implications of echocardiographically assisted diagnosis of pericardial tamponade in contemporary medical patients: detection before hemodynamic embarrassment. J Am Coll Cardiol 17: 59-65. [Crossref]
-
7. Appleton CP, Hatle LK, Popp RL (1988) Cardiac tamponade and pericardial effusion: respiratory variation in transvalvular flow velocities studied by Doppler echocardiography. J Am Coll Cardiol 11: 1020-1030. [Crossref]
-
8. Sagrista-Sauleda J, Merce AS, Soler-Soler J (2011) Diagnosis and management of pericardial effusion. World J Cardiol 3: 135-143. [Crossref]
-
9. Palacios IF, Tuzcu EM, Ziskind AA, Younger J, Block PC (1991) Percutaneous balloon pericardial window for patients with malignant pericardial effusion and tamponade. Cathet Cardiovasc Diagn 22, 244-249. [Crossref]
-
10. Hazelrigg SR, Mack MJ, Landreneau RJ, Acuff TE, Seifert PE, et al. (1993) Thoracoscopic pericardiectomy for effusive pericardial disease. Ann Thorac Surg 56: 792-795. [Crossref]
-
11. Chambliss JR, Jaruszewski EJ, Brofman BL, Martin JF, Feil H (1951) Chronic cardiac compression (chronic constrictive pericarditis); a critical study of sixty-one operated cases with follow-up. Circulation 4: 816-835. [Crossref]
-
12. Kussmaul A (1873) Ueber schwielige Mediastino-Pericarditis und den paradoxen. Puls.Klin.Wochenschr 10: 445.
-
13. Myers RB, Spodick DH (1999) Constrictive pericarditis: clinical and pathophysiologic characteristics. Am Heart J 138: 219-232. [Crossref]
-
14. Mangi AA, Torchiana DF (2012) Pericardial Disease, Cardiac Surgery in the Adult. McGraw Hill Education, USA, 1465-1478.
-
15. Santamore WP, Bartlett R, Van Buren SJ, Dowd MK, Kutcher MA (1986) Ventricular coupling in constrictive pericarditis. Circulation 74: 597-602. [Crossref]
-
16. Sechtem U, Tscholakoff D, Higgins CB (1986) MRI of the abnormal pericardium. AJR Am J Roentgenol 147: 245-252. [Crossref]
-
17. Aroney CN, Ruddy TD, Dighero H, Fifer MA, Boucher CA, et al. (1989) Differentiation of restrictive cardiomyopathy from pericardial constriction: assessment of diastolic function by radionuclide angiography. J Am Coll Cardiol 13: 1007-1014. [Crossref]
-
18. Hurrell DG, Nishimura RA, Higano ST, Appleton CP, Danielson GK, et al. (1996) Value of dynamic respiratory changes in left and right ventricular pressures for the diagnosis of constrictive pericarditis. Circulation 93: 2007-2013. [Crossref]
-
19. Oh JK, Hatle LK, Seward JB, Danielson GK, Schaff HV, et al. (1994) Diagnostic role of Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol 23: 154-162. [Crossref]
-
20. Rajagopalan N, Garcia MJ, Rodriguez L, Murray RD, Apperson-Hansen C, et al. (2001) Comparison of new Doppler echocardiographic methods to differentiate constrictive pericardial heart disease and restrictive cardiomyopathy. Am J Cardiol 87: 86-94. [Crossref]
-
21. Young PM, Glockner JF, Williamson EE, Morris MF, Araoz PA, et al. (2012) MR imaging findings in 76 consecutive surgically proven cases of pericardial disease with CT and pathologic correlation. Int J Cardiovasc Imaging 28: 1099-1109. [Crossref]
-
22. Lange RL, Botticelli JT, Tsagaris TJ, Walker JA, Gani M, et al. (1966) Diagnostic signs in compressive cardiac disorders. Constrictive pericarditis, pericardial effusion, and tamponade. Circulation 33: 763-777. [Crossref]
-
23. Imazio M, Cecchi E, Demichelis B, Ierna S, Demarie D, et al. (2007) Indicators of poor prognosis of acute pericarditis. Circulation 115: 2739-2744.
-
24. Imazio M, Spodick DH, Brucato A, Trinchero R, Adler Y (2010) Controversial issues in the management of pericardial diseases. Circulation 121: 916-928. [Crossref]
-
25. Imazio M, Demichelis B, Parrini I, Giuggia M, Cecchi E, et al. (2004) Day-hospital treatment of acute pericarditis: a management program for outpatient therapy. J Am Coll Cardiol 43: 1042-1046. [Crossref]
-
26. Permanyer-Miralda G (2004) Acute pericardial disease: approach to the aetiologic diagnosis. Heart 90: 252-254. [Crossref]
-
27. Imazio M, Trinchero R (2008) Myopericarditis: Etiology, management, and prognosis. Int J Cardiol 127: 17-26. [Crossref]
-
28. Soler-Soler J, Sagrista-Sauleda J, Permanyer-Miralda G (2004) Relapsing pericarditis. Heart 90: 1364-1368. [Crossref]
-
29. McDonald JM, Meyers BF, Guthrie TJ, Battafarano RJ , Cooper JD, et al. (2003) Comparison of open subxiphoid pericardial drainage with percutaneous catheter drainage for symptomatic pericardial effusion. Ann Thorac Surg 76: 811-815. [Crossref]
-
30. Augustin P, Desmard M, Mordant P, Lasocki S, Maury JM, et al. (2011) Clinical review: intrapericardial fibrinolysis in management of purulent pericarditis. Crit Care 15: 220. [Crossref]
-
31. Bennett EV Jr. (1984) Purulent pericarditis. J Thorac Cardiovasc Surg 87: 641-642.
-
32. Diacon AH, Theron J, Schuurmans MM, Van de Wal BW, Bolliger CT (2004) Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med 170: 49-53. [Crossref]
-
33. Tsang TS, Califf RM, Stebbins AL, Lee KL, Cho S, et al. (1997). Incidence and impact on outcome of streptokinase allergy in the GUSTO-I trial. Global Utilization of Streptokinase and t-PA in Occluded Coronary Arteries. Am J Cardiol 79: 1232-1235. [Crossref]
-
34. Majid AA, Omar A (1991) Diagnosis and management of purulent pericarditis. Experience with pericardiectomy. J Thorac Cardiovasc Surg 102: 413-417. [Crossref]
-
35. Sethi GK, Nelson RM, Jenson CB (1973) Surgical management of acute septic pericarditis. Chest 63: 732-735. [Crossref]
-
36. Bansal R, Perez L, Razzouk A, Wang N, Bailey L (2010) Pericardial constriction after cardiac transplantation. J Heart Lung Transplant 29: 371-377. [Crossref]
-
37. Nataf P, Cacoub P, Dorent R, Jault F, Bors V, et al. (1993) Results of subtotal pericardiectomy for constrictive pericarditis. Eur J Cardiothorac Surg 7: 252-255. [Crossref]
-
38. Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P , Ozduran V, et al. (2004) Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 43: 1445-1452. [Crossref]
-
39. Warren WH (2000) Malignancies involving the pericardium. Semin Thorac Cardiovasc Surg 12: 119-129. [Crossref]
-
40. Lam KY, Dickens P, Chan AC (1993) Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med 117: 1027-1031. [Crossref]
-
41. Patel J, Park C, Michaels J, Rosen S, Kort S (2004) Pericardial cyst: case reports and a literature review. Echocardiography 21: 269-272. [Crossref]
-
42. Najib MQ, Chaliki HP, Raizada A, Ganji JL, Panse PM, et al. (2011) Symptomatic pericardial cyst: a case series. Eur J Echocardiogr 12: E43. [Crossref]


