Cardiopulmonary bypass surgery has been implicated in causing atelectasis; a major cause of intrapulmonary shunting and hypoxemia postoperatively. This study investigated if repetitive lung recruitment manoeuvres (RRM), before and after extubation, could reduce intrapulmonary shunting for a longer period post-extubation than only one standardized recruitment manoeuvre (SRM),
Forty cardiac valve replacement patients were randomised after SRM into two groups: RRM group (n=20): Total vital capacity manoeuvre at surgery end and repeated every 4 hours until extubation; SC group (n=20): Standard care with SRM. Intrapulmonary shunts (Qs/Qt) were measured after anaesthesia induction, at surgery termination and every 4 hours until 24 hours post-extubation. Time to extubation was recorded. Lung function was measured every 12 hours until discharge.
A 24 hour post-extubation ANOVA showed no Qs/Qt significant differences between RRM and SC group (4.5 ± 3.3% and 5.3 ± 2.4%). At surgery end, Qs/Qt increased in RRM group (7.3 ± 3.2% to 13.5 ± 3.7%; p<10-3) but was significantly less (p<0.02) compared to SC group (7.3 ± 3.7% to 16.1 ± 6%; p<10-3). Time to extubation was 8.8 ± 4.2 h in RRM group versus 10.4 ± 4.3 h SC group (p<0.2, ns). No significant difference in vital capacity was seen in the RRM group (3.17 ± 1.2 to 1.9 ± 1 l) compared to SC group (3.4 ± 0.8 to 2.1 ± 0.9 l).
In valve replacement surgery, RRM is only beneficial in the short-term period and does not assure a better intrapulmonary shunt benefit than one SRM applied at the end of surgery.
Pulmonary complications are relatively frequent after cardiac surgery and remain the major cause of morbidity and mortality in the post-surgery period [1-10]. Pulmonary atelectasis (condition of deflated alveoli/collapsed lung) is the most common complication after this type of surgery [2,4,11]. The incidence of atelectasis varies between 20 to 75% (12). A scannographic study showed that atelectasis affects 24% of the pulmonary surface and predisposes patients to pulmonary infections and to other problems, such as difficulty in weaning patients from the ventilator in the post-surgery period [13]. Atelectasis explains 67% of the pulmonary shunt effect, which is considered the major pathophysiological mechanism for post-surgical arterial hypoxemia [7,11,14]. Tschernko et al. have proved that pulmonary shunt increases to around 16.8 ± 6.7% at the end of cardiopulmonary bypass (CPB), and continues to increase in the post-extubation period [15]. Four hours after extubation, the shunt was shown to be around 25.6 ± 8.1%. This result was confirmed by a recent study which showed the shunt as 30.3 ± 14.1% [16]. Moreover, Bender et al. showed that the pulmonary shunt can still be high at 24 hours after extubation (29 ± 12.3%) [17].
Several types of medical intervention are employed to decrease the risk of atelectasis [18-24]. currently the most widespread used method is incentive spirometry, although the majority of studies do not show it to have a significant effect (21). Another post-operative intervention to recruit collapsed alveoli is the multiple hyperinflation technique, which is sub-divided into 2 groups: low pressure techniques (CPAP; BiPAP) and high pressure (IPPB [19,20,22,23,25]. However, in spite of their tempting concept and their effectiveness in small open studies, meta-analysis of randomized and controlled studies has not found a significant benefit [23].
In recent years at the end of cardiopulmonary bypass (CPB) surgery and before patient extubation, alveolar recruitment manoeuvres were shown to have some benefit on post-surgical hypoxia and allowed an earlier extubation in the treated group [5,15,18,22,26-28]. The total vital capacity manoeuvre (TVCM) is standardized and is the most commonly used. It consists of inflating the lungs for 15 seconds’ duration and maintaining the airway pressure to a level of 40 cm H2O [29]. The studies were restricted by the limited number of patients and short term benefits gained [5,15,27,28]. Minkovich et Coll showed that two consecutive recruitment manoeuvres result in a better arterial oxygenation extending from the immediate postoperative period to approximately 24 hours after surgery [26]. In a recent study, Leme et al demonstrated that the use of an intensive vs a moderate alveolar recruitment strategy resulted in less severe pulmonary complications [18].
This study assesses the efficacy of a new therapeutic approach, which aims to prevent deterioration of pulmonary function secondary to atelectasis and thereby limits pulmonary complications after cardiac surgery, compared to a standard approach. This new therapeutic approach called repetitive recruitment manoeuvre (RRM) begins directly at the end of the surgery, after closing the sternum, and is maintained until 24 hours post-extubation. It aims to prolong the gas exchange benefit of a standard care including standard recruitment manoeuvre (SRM) applied before separation from CPB. Our primary objective was to study the benefit of RRM on postoperative hypoxemia using pulmonary shunt. Additionally, the alveolo-arterial oxygen gradient, the effects of RRM on the time to extubation, lung function aqnd pulmonary complications were determined.
Patients
This study was conducted in the cardiac surgery unit of the Hôtel-Dieu de France university hospital in Beirut, Lebanon, linked to Saint-Joseph University. The unit of cardiac intensive care has six beds for thoracic surgery patients. The study was conducted on 40 ICU patients over a period of one year. Institutional review board approval and written informed consent were obtained for all patients. Inclusion criteria included patients who had to be undergoing extracorporeal circulation (ECC) for a valve replacement (VR). Patients who needed a cardiopulmonary bypass for an aortocoronary bypass were not allowed to participate in the study for eminent reasons related to the bypass itself. Exclusion criteria included: Age >80 years old; heart failure with left ventricular ejection fraction <40%; obese patients with body mass index >30; recent pulmonary oedema in the pre-operative period (<one month); Significant obstructive or restrictive lung disease (vital capacity (VC) <70% and/or forced expiratory volume in one second (FEV1) <70%). Patients diagnosed with obstructive disease received an optimal respiratory broncho-dilatator treatment that combined salbutamol and ipratropium. All patients signed a consent form once they had satisfied the eligibility criteria. A computerized randomisation was used to assign patients to their relative groups. Any critical events had to be notified as well as patient compliance.
Applied methods
Both groups: Patients were premedicated with hydroxyzine 1 mg kg-1. General anaesthesia was induced using propofol 2 mg kg-1, fentanyl 1µg kg-1 and succinylcholine 1 mg kg-1. After tracheal intubation, anaesthesia was maintained with one MAC (minimum alveolar concentration) sevoflurane and fentanyl 1-2 µg kg-1 h-1 .Neuromuscular blockade was obtained by cisatracurium with a loading dose of 0.2 mg kg-1 followed by a continuous infusion of 2-4 µg kg-1 h-1 .Anaesthesia drugs were calculated on an ideal body weight basis. Intraoperative monitoring included five-lead electrocardiography, invasive radial arterial pressure, pulmonary artery catheter, pulse oximetry, capnography, urine output and rectal temperature. All patients were mechanically ventilated (Datex-ohmeda Aestiva/5; Helsinski, Finland) with a tidal volume of 8 ml kg-1 of ideal body weight, an inspiratory/expiratory time ratio of 0.4 and a mixture of oxygen (O2) and air adjusted to have a fixed inspired oxygen concentration of 40%. End-tidal carbon dioxide (ETCO2) was continuously monitored and respiratory rate was subsequently adjusted to maintain ETCO2 at a level of 30-35 mm Hg.
At the end of surgery (I) and before separation from CPB, a standard recruitment manoeuvre (SRM) was applied at the end of surgery in both groups and before sternum closure. SRM was standardized to receive sustained manual inflation for a period of eight seconds at a pressure of 40 cm H2O. The SRM was followed by a passive expiration without pause, and repeated three times. The study procedural timeline is shown in Table 1.
Table 1. Procedural Timeline
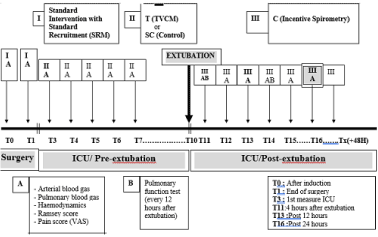
After surgery, all patients were transferred from the operating room to the ICU while receiving manual ventilation using an Ambu-type resuscitation bag (Ambu SPUR; Ambu Inc, Lithicum, MD, USA). On ICU admission, mechanical ventilation of the lungs was started with a 7600 Ventilator System (Nellcor Puritan Bennett Inc, Pleasanton, CA, USA) using assist-control mode. Initial ventilator parameters were set at a tidal volume of 8 ml kg-1, a respiratory rate of 14 /min, PEEP of 5 cm Hg, an inspiratory trigger of 0.3 cm H2O and an FiO2 of 0.4.
Pressure support mode of ventilation (PSV) was applied to wean all patients from mechanical ventilation. Extubation was performed if the patient was haemodynamically stable, fully awake, with no signs of respiratory distress and/or clinically significant hypoxemia after one hour of minimal respiratory support (5 cm H2O and PEEP of 5 cm H2O). Table 1. Procedural Timeline
Repetitive recruitment manoeuvre (RRM) group: RRM was applied in the pre-extubation phase (II) and the post-extubation phase (III). In the pre-extubation phase (II) patients had a total vital capacity manoeuvre (TVCM) every four hours and for as long as they remained intubated. TVCM involved lung inflation for a period of 8 seconds at a pressure of 40cm H2O. The first TVCM in the treated group was performed at the end of surgery in the operating room just after closing the sternum (T1), and then repeated four hours later in the ICU (T3: first measure in the ICU) and every 4 hours until extubation. This manoeuvre was repeated three times in each session at five minutes’ intervals.
Standard Care (SC) Control group: Incentive spirometry (Portex Inc. Keene, NH 03431, USA) was applied in the post-extubation period in the SC group. The inspiratory dial setting was fixed at two and expiratory dial setting at four. The session duration was of three minutes, and was repeated six times a day at fixed hours.
Measurements
- Oxygenation (Shunt and A-a gradient) and haemodynamics: Arterial and pulmonary blood gas and haemodynamics were measured after intubation and repeated at each four hourly-period mentioned above. Measurements were made 15 minutes after the end of the manoeuvre (TVCM). The principal evaluation was made on the degree of oxygenation at 24 hours, evaluated by the intrapulmonary shunt (Qs/Qt) (% of cardiac index) (Appendix). Secondary evaluation criteria were: alveoli-arterial oxygen gradient (Δ (A-a)) (mm Hg) at 24 hours and 48 hours, pulmonary shunt at 48 hours (Appendix).
- Respiratory Mechanics: Complete pulmonary function tests were initially performed at the pre-operative baseline using both a non-mobile unit (Collins-GS, USA) and a portable unit (Creative Biomedics, USA). The portable device was used while the patient was still in the ICU. Vital capacity (VC), forced expiratory volume in one second (FEV1) and forced expiratory fraction (FEF) 25-75% were measured after extubation and at each 12-hour time point up until 48 hours after surgery. To determine lung volume, a lung function test using a helium technique was made prior to discharge from hospital.
- Surgery time: start of intubation until sternum closure.
- Time to extubation (hours) were determined. Time of discharge from the intensive care unit and from hospital were recorded
- Pulmonary complications such as pneumonia, pleural effusion, pneumothorax, SDRA., re-intubation or need of ventilation were recorded as unexpected events
While patients were intubated, measurements were systematically made fifteen minutes after TVCM in the RRM group, but every four hours in the control SC group. In the post-extubation period, measurements were made fifteen minutes after IPPB and incentive spirometry (IS) dependant of the group.
Statistics
Sample size: Patients who had cardiac surgery involving a CPB were shown to have an intrapulmonary shunt increase since anaesthesia induction. Bender et al. showed that the shunt was still high at 24 hours after extubation (29±12.3%) [17]. RRMs were hypothesized to decrease the pulmonary shunt for a period as long as 24 hours. A decrease in intrapulmonary shunt from 29 to 19% (~1/3) is considered clinically significant. Assuming a power of 80% and type I error of 5% and a unilateral test, the number of study patients required in each group was 20 with a study total of 40.
Statistical analysis: Data are presented as mean ±SD for continuous parameters and as percentages for categorical parameters. Continuous variables are checked for normality. Quantitative parameters within the same group were compared at two different time points with a paired Student t-test. Changes within the same group were studied using one-way analysis of variance (ANOVA). The comparison of changes for the examined parameters (respirator mechanics, haemodynamics, intrapulmonary shunt and other gasometrical parameters) between RRM and SC groups were performed using two-way ANOVA.
The statistical software used in this study was SPSS v23 (SPSS Inc, Chicago, Illinois, USA).
Baseline
Forty subjects were randomized into two study groups. The ratio of male to female patients were split 21:19 male to female. Groups were comparable with respect to demographics, left ventricular and lung functions data (Table 2).
Table 2. Group Demographic Data
BMI = Body mass index; LVEF = Left ventricular ejection fraction; VC = Vital capacity; FEV1 = Forced expiratory volume in one second
|
RRM group
(n=20) |
Control group
(n= 20) |
Age (Years) |
55.8 ± 13.9 |
57.5 ± 15.1 |
Weight (Kg) |
71.6 ± 15.3 |
76.2 ± 14.6 |
Height (cm) |
164.7 ± 11.1 |
168.7 ± 11 |
BMI (Kg/m2) |
26.3 ± 4.3 |
26.9 ± 5.6 |
LVEF (%) |
66.8 ± 5.5 |
62.2 ± 6.5 |
VC0 (litre) |
3.281 ± 1.211 |
3.287 ± 1.124 |
FEV10 (litre) |
2,702 ± 1.027 |
2,865 ± 1.074 |
CV0 (%) |
91.4 ± 16.5 |
93.4 ± 14.7 |
FEV10 (%) |
98.4 ± 15.6 |
98.3 ± 16.5 |
The types of valve replacement were similarly distributed between the two study groups with the mitral valve replacement being the commonest surgery (12 of 20 in the RRM group and 15 of 20 in the SC group). Other surgical treatment data, such as duration of surgery, duration of CPB and aortic cross clamping were also comparable (Table 3).
Table 3. Treatment data
Group |
RMM group |
Control group |
| |
(n=20) |
(n=20) |
Valve (n) |
|
|
Aortic |
6 |
4 |
Mitral |
12 |
15 |
Mitral-Aortic |
1 |
1 |
Mitral-Tricuspid |
1 |
0 |
Surgery time (min) |
225 ± 61.3 |
232 ± 47.1 |
ECC (min) |
92.9 ± 21.2 |
93.4 ± 27.8 |
ACC (min) |
73.4 ± 17.2 |
74.2 ± 20.1 |
Mechanical ventilation (min) |
813.4±254.7 |
905 ± 258.7 |
Time to extubation (min) |
533.4 ± 252 |
625.2 ± 258 |
Ramsey score (ICU admission) |
1.75 ± 1.1 |
1.82 ± 1.4 |
Pain –VAS (mm)
( Post extubation) |
35 ± 14 |
37 ± 16 |
ECC= Extracorporal circulation; ACC = Aortic cross clamping; VAS = Visual analog scale
Systemic and pulmonary haemodynamics outcome
Parameters characterizing systemic and central haemodynamics are presented in Table 4. These parameters were evaluated after induction of anaesthesia (baseline), at the end of surgery (after sternum closure) and 4, 12 and 24 hours after extubation. No haemodynamic abnormalities were observed before, during or after surgery between the two groups. Heart rate remained stable after surgery and extubation in both groups. Cardiac index (CI) increased in both groups after surgery (p< 0.05). PCWP (wedge pressure), SVRI (systemic vascular resistance index) and PVRI (pulmonary vascular resistance index) decreased significantly in both groups (p< 0.05). Only PVRI increased at the end of surgery compared to baseline in both groups (p< 0.05). Mean arterial pressure, central venous pressure and mean pulmonary arterial pressure remained unchanged.
Table 4. Systemic and central hemodynamic data
MAP= Mean arterial pressure; MPAP= Mean pulmonary arterial pressure; PCWP= wedge pressure; CI = Cardiac index; SVRI = Systemic vascular resistance indexed; PVRI = Pulmonary vascular resistance indexed; T0 = After induction ; T1= At the end of surgery ; T11: After extubation; T13 = 12 hour after extubation; T16= 24 hours after extubation
*p < .05 compared to baseline state; †p < .05 compared to the control group
Group |
Time |
RMM group
(n=20) |
SC (control) group
(n= 20) |
MAP
(mm Hg) |
T0
T1
T11
T13
T16 |
75.9 ± 12.6
85 ± 13.2
81 ± 7.8
78.3 ± 8.5
82.1 ± 10.2 |
76.4 ± 12.9
87.5 ± 15.4
79.7 ± 6
78.1 ± 8.3
80.3 ± 10.6 |
MPAP
(mm Hg) |
T0
T1
T11
T13
T16 |
18.7 ± 6.3
18.5 ± 5.3
18.4 ± 7.5
18 ± 7.9
18.3 ± 4.8 |
22.3 ± 5.9
20.8 ±6.3
17.3 ± 5.2
16.7 ± 2.7
20.4 ± 6.1 |
PCWP
(mm Hg) |
T0
T1
T11
T13
T16 |
14.5 ± 5.2
12.2 ± 4*
10.4 ± 5*
10.9 ± 4.7*
10.5 ± 3.6* |
15.8 ± 4.9
13.1 ± 4.4*
9.5 ± 4.5*
9.9 ± 3.5*
12 ± 2.6* |
CI
(L.min-1.m-2) |
T0
T1
T11
T13
T16 |
2.1 ± 0.8
2.4 ± 1
3.4 ± 0.7*
3.1 ± 0.7*
3.4 ± 0.7* |
2 ± 0.5
2.2 ± 0.6
3 ± 0.6*
3 ± 0.4*
3.4 ± 0.6* |
SVRI |
T0
T1
T11
T13
T16 |
2867 ± 989
2657 ± 1151
1020 ± 227*
1179 ± 308*
1164 ± 346* |
2957 ± 898
2942 ± 1141
1193 ± 267*
1344 ± 317*
1139 ± 276* |
PVRI |
T0
T1
T11
T13
T16 |
193 ± 76
233 ± 151
106 ±42*
119 ± 85*
129 ± 76* |
185 ± 84
230 ± 142
160 ± 116
199 ± 104
129 ± 83* |
Gas exchange outcome
At surgery end, Qs/Qt increased in RRM group (7.3 ± 3.2% to 13.5 ± 3.7%; p<10-3) but was significantly less (p<0.02) compared to SC group (7.3 ± 3.7% to 16.1 ± 6%; p<10-3). For the primary outcome, an ANOVA at 24 hours post-extubation showed a Qs/Qt of 5.3 ± 2.4% for the RRM group and 4.5 ± 3 for the SC group. When the intrapulmonary shunt of the RRM group was compared to the SC group they were found to be similar (Figure 1 and 2).
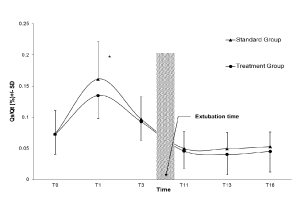
Figure 1. RRM treatment group versus Standard Care Group: Effect on intrapulmonary shunt.
T0 = After induction ; T1= At the end of surgery ; T3= first measure in the ICU (4 hour after the end of surgery);T11: first measure after extubation; T13 = 12 hour after extubation; T16= 24 hours after extubation
* p < .05 compared to the control group
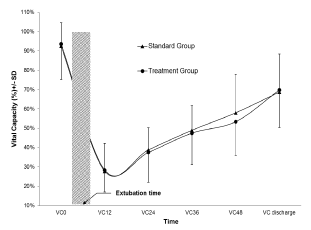
Figure 2. RRM treatment group versus Standard Care Group: Effect on lung function (vital capacity)
VC0 = Baseline ; VC12= 12 hours after extubation ; VC24 = 24 hours after extubation ; VC36 = 36 hours after extubation ; VC48 = 48 hours after extubation ; VC discharge = Discharge from the hospital
When the alveolo-arterial oxygen gradient of the RRM group was compared to the SC group, it was found that their progression was parallel (p= ns). In both groups, gradient increased from baseline to the end of the surgery and then decreased up until 24 hours post-extubation in RRM (132 ± 63 and 81 ± 53; p< 0.05) and in SC group (134 ± 75 and 94 ± 43; p< 0.05) respectively. Difference in arterial partial pressure of oxygen was significantly higher in RMM group compared to SC group at T0 (256 ± 49 and 218 ± 78 mm Hg; p< 0.05) and T1(424 ± 87 and 329 ± 131 mm Hg; p< 0.05) respectively, disappeared from T3 period.
Ventilatory and lung function outcome
Lung function was significantly reduced (p< 0.05) after extubation in both groups compared to baseline with a measured nadir just after extubation (28.3% ± 18.4% in RRM and 27.7% ± 12.2%) and persists thereafter (Fig 2). No significant difference in vital capacity was seen in the RRM group (3.17 ± 1.2 to 1.9 ± 1 litre) compared to SC group (3.4 ± 0.8 to 2.1 ± 0.9 litre) at 24 hours after extubation. At hospital discharge, lung function continued to recuperate progressively in both groups and reached 69.8% ± 19.4% in the RRM group and 68.7% ± 19.6% in the SC group (p> 0.05).
Clinical outcome
Time to extubation showed a tendency to be shorter in the RRM group (8.8 ± 4.4 hours) compared to the SC group (10.4 ± 4.3 hours) respectively (p= 0.1, ns). Duration of ICU stay was the same in the 2 groups (36.6 ± 5.2 hours in RRM group and 38.7 ± 4.6 hours in the control group, (p= ns). None of the patients of the treated and controlled groups required an ICU stay longer than 2 days. The duration of hospital stay after surgery was identical in the treated group and the control group (4.8 ± 1.6 days and 5.1 ± 1.8 days respectively; p = ns)
Sedation and pain scores were comparable among both groups after the operation (Table 3). All prefixed sessions of recruitment or incentive spirometry were accomplished. All recruitment manoeuvres were well tolerated without any major reported complications. Only transient and spontaneously resolved arterial hypotension lasting a few seconds (< 15% of baseline value) encountered in four of the 20 patients in the TVCM phase. Patients in RRM group cooperated well with settled TVCM in pre-extubation phase and IPPB after extubation.
The SRM applied at the end of cardiac surgery, before sternum closure, was of great benefit in limiting the normal increase of Qs/Qt in both groups. RRM after this standard manoeuvre had a significant but only transient add-on effect (4 hours) on shunting. Medium term benefit (24-hour post extubation) of ongoing RRM on shunting was rapidly lost with time and was comparable to standard care. The progression in shunting was associated with a parallel effect on oxygenation post-operatively.
Increased Qs/Qt is a well-documented and undesirable phenomenon after CPB in cardiac surgery patients [5,11,13,14,16,26,30]. The effects of pulmonary atelectasis during CPB on post-CPB shunting and oxygenation is well demonstrated in animals and humans and is seen especially at the end of the surgery [11,15.26]. Even though the precise mechanisms of CPB related acute lung injury are still unknown, a formation of collapsed unstable alveolar units is the final outcome of this type of lung injury that clinically manifests as atelectasis, increased intrapulmonary shunt and hypoxemia [4,7,9,11,14,31].
The recruitment manoeuvre using the vital capacity manoeuvre (TVCM) performed before the end of bypass was effective in preventing post-operative impairment in gas exchange [15,18,26,28,32]. In fact, Rothen et al. recently proved that this manoeuvre needs to be maintained for 8 seconds only to allow a re-expansion of packed pulmonary tissue and was not necessary for 15 seconds as described previously [33]. However, Murphy et al. showed that the benefits are quickly lost after patient extubation [27]. Moreover, Tschernko et al showed that even with TVCM the shunt returned to control values after extubation (10.7 ± 4.3 at the end of surgery to 24.4 ± 8.5% after extubation) [15]. In contrast, Minkovich et al found in a recent randomized controlled trial that two recruitment manoeuvres: the first applied at the end of surgery before CPB separation and the second on arrival to the ICU, can maintain the benefits on oxygenation ratio extending from the immediate post-operative period to approximately 24 hours after surgery at the time of ICU discharge [26]. No additional benefit is found in our study by repeating recruitment manoeuvre in valvular replacement surgery if done after a well-executed standard recruitment manoeuvre (SRM) applied before CPB termination.
After a SRM used routinely in all patients, the usual increase of Qs/Qt post-surgery in CPB in both study groups was mild (peak value of 16.1 ± 6% in RRM group and 13.5 ± 3.7% in SC group at the end of surgery (T1) and resolved rapidly after 4 hours (T3) even before extubation. Intrapulmonary shunt after cardiac surgery was reported to vary from around 16% at the end of surgery, increased fastly after 4 hours and reached 29% at 24 hours [15-17]. Our sample size was calculated to reduce intrapulmonary shunt gradient from 29% to 19% in RMM compared to SC. These discrepancies can be due to multiple reasons. First, SRM was applied in our study at the end of surgery with the sternum still open. This timing can participate to a better outcome which facilitates the alveolar recruitment without the restriction of the chest wall; secondly, SRM followed a standardized method of recruitment which targeted 40 cm H2O with an individualized vital capacity using manual Ambu-type resuscitation bag with a manometer; thirdly, this manoeuvre was repeated for three complete cycles. Unfortunately, in our study, a third group without the SRM performed before the end of surgery could have been included and would have helped to calculate the particular effect of this manoeuvre in our SC group. In both groups, no need for re-exploration for bleeding was required and no clinical significant adverse hemodynamic effects in our study [1,3,15-17].
Post-operative conventional volume target ventilation with a low level of PEEP (5 cm H2O) was applied in the two study groups. This mode of ventilation with its low PEEP was well tolerated. It can be hypothesized that this low PEEP can slowly reopen partially collapsed lung tissue especially after a well performed recruitment manoeuvre. This explains the well-established spontaneous slow improvement in gas exchange seen in animal studies [5,11,16]. In a recent study, Hajjar et al showed that pressure-controlled ventilation with recruitment resulted in better oxygenation than volume-controlled ventilation [34].
Pulmonary consequences after CBP may differ depending on the type of cardiac surgery. It remains unclear to what extent differences between coronary artery bypass graft surgery (CABG) or valve replacement with CPB can influence these lung complications. In our study, surgeons were concerned about any potential effect of the hyperinflated lung on the graft especially when internal mammary artery bypass was used. Tschernko et al. [15] found that in CABG patients, intrapulmonary shunt increased from the end of surgery to four hours after extubation (16.8 ± 6.7% to 25.6 ± 8.1%). Although using the same CPB and ventilation technique, this discrepancy is hypothesized to occur in CABG patients compared to valve replacement patients. In CABG surgery, the technical aspects of implanting the venous grafts on the coronary vessels per se may result in a harmful consequence on lung function by inducing alveolar collapse. This is due in part to the necessity of opening the pleural cavity, but also due to compression of the lung while the surgeon facilitates visualization of the operational area. This is of special note when the internal mammary artery is used, and may explain a major part of the high shunt levels seen in these patients. In our study, we included only valve replacement candidates. CABG candidates were excluded because surgeons were concerned of a potential harmful effect of the manoeuvre on the coronary vascular grafts. However, these results should be assessed before extrapolating these finding to CABG patients.
In our controlled and randomised study, we thought that repeating the recruitment sessions for a longer period, even after extubation, would result in a better outcome. Pasquina et al found that non-invasive pressure-support ventilation applied four times a day for 30 minutes, after tracheal extubation, preserves oxygenation in patients after cardiac surgery [35]. RRM with TVCM while still intubated and IPPB after extubation made in the standard manner did not result in any further benefit on gas exchange and oxygenation. These results are consistent with the study of Manugssen et al. who showed that repetitive manoeuvres in animals, even when proceeding sternum closure, gave the same benefit as TVCM alone [5]. It seems that the significant add-on effect noted after surgery (13.5 ± 3.7% in RRM group compared to 16.1 ± 6% in the SC group; p <10-3) was rapidly lost 4 hours after surgery, while the patient was still on mechanical ventilation, and continues thereafter before and after extubation parallel to the SC group [36]. As a consequence, the primary outcome Qs/Qt at 24 hours post-extubation showed no significant differences (5.3 ± 2.4% for the RRM group and 4.5 ± 3 for the SC group respectively).
Acute restrictive pulmonary deficits are well documented sequelae of cardiac surgery [30,37]. Decreased pulmonary compliance, surgical incision, sedation and pain might be partially responsible for altered tidal volume. The first measured vital capacity after extubation was the lowest in the two groups (28.3% ± 11.2% in RRM and 27.7% ± 14.5 in the SC group) and reached only two thirds of baseline values at hospital discharge. For the vital capacity to return to baseline at least eight weeks were needed [26,37].
Increased CI as well as decreased SVRI in patients undergoing cardiac surgery with extracorporal circulation are comparable to the findings in other studies, and can have two explanations [15,38,39]. Firstly, the correction of valvular dysfunction plays a major role in the amelioration of cardiac performance and hence stroke volume. Secondly, it is likely that the use of the extracorporal circuit is associated with decreased SVRI leading to increased CI. However, the increase in blood flow has little effect on pulmonary blood volume in normal lungs and unlikely to be attributed to the impairment of lung function [39,40]. On the other hand, we cannot comment on changes in lung water because we did not measure this parameter. Nevertheless, PCWP decreased after cardiac surgery and did not seem to be responsible for impaired oxygenation [41,42]. In summary, differences in haemodynamics did not seem responsible for increased intrapulmonary shunting in our patients.
Although a TVCM did not influence pulmonary gas exchange in the ICU, its application in the operating room appears to exert a beneficial effect on tracheal extubation times after cardiac surgery, Murphy et al showed that TVCM patients were extubated earlier than the control group (6.5 ± 2.1 hours’ vs 9.4 ± 4.2 hours; p = 0.01) [27]. Berry et al found that PEEP applied during surgery didn’t succeed to modify the extubation time [38]. In our study, extubation times in both groups were not statistically significant with RRM group (8.8 ± 4.4 hours) and SC group (10.4 ± 4.3 hours) respectively.
We conclude that after CPB surgery for valve replacement, repetitive lung recruitment manoeuvres (RRM) applied every four hours after a single standard respiratory manoeuvre (SRM) assure only short term benefit. RRM does not assure a better oxygenation or sustained intrapulmonary shunt benefit than a standardised single recruitment manoeuvre applied at the end of surgery with an open sternum.
I would like to dedicate this work for the memorial of my friend and co-author Prof Alexandre Yazigi. His passion and patience has left an impact on me that I will never wear out.
1. Intrapulmonary shunt: Qs/QT = (CcO2 – CaO2) / (CcO2 – CvO2)
CcO2 = end-capillary oxygen content
CaO2 = arterial oxygen content
CvO2 = mixed venous oxygen content
2.Alveolar-arterial oxygen gradient:
P (A-a) = PAO2 – PaO2
PAO2 = FIO2 (PB – PH2O) – PaCO2 [FiO2 + (1-FiO2)/RG]
PAO2 = alveolar PO2
PaO2 = arterial oxygen
PaCO2 = arterial carbon dioxide
FiO2 = inspired oxygen concentration
PB = barometric pressure
PH2O = water vapor pressure
RQ = respiratory quotient (0.8)
- Bautin AE, Mazurok VA, Osovskikh VV, Afanas'eva KIu (2014) [Hemodynamic effects of the alveolar recruitment maneuver in cardiosurgical patients with left ventricular systolic dysfunction]. Anesteziol Reanimatol 59: 43-48. [Crossref]
- Canver CC, Cooler SD, Nichols RD (1998) The influence of cardiopulmonary function on outcome of veterans undergoing resectional therapy for lung cancer. J Cardiovasc Surg (Torino) 39: 497-501. [Crossref]
- De Broca B, Garnier J, Fischer MO, Archange T, Marc J, et al. (2016) Stroke volume changes induced by a recruitment maneuver predict fluid responsiveness in patients with protective ventilation in the operating theater. Medicine (Baltimore) 95: e4259. [Crossref]
- Magnusson L1, Spahn DR (2003) New concepts of atelectasis during general anaesthesia. Br J Anaesth 91: 61-72. [Crossref]
- Magnusson L1, Zemgulis V, Tenling A, Wernlund J, Tydén H, et al. (1998) Use of a vital capacity maneuver to prevent atelectasis after cardiopulmonary bypass: an experimental study. Anesthesiology 88: 134-142. [Crossref]
- Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, et al. (1996) Atelectasis and pulmonary shunting during induction of general anaesthesia--can they be avoided? Acta Anaesthesiol Scand 40: 524-529. [Crossref]
- Tenling A1, Hachenberg T, Tydén H, Wegenius G, Hedenstierna G (1998) Atelectasis and gas exchange after cardiac surgery. Anesthesiology 89: 371-378. [Crossref]
- Tokics L1, Hedenstierna G, Svensson L, Brismar B, Cederlund T, et al. (1996) V/Q distribution and correlation to atelectasis in anesthetized paralyzed humans. J Appl Physiol (1985) 81: 1822-1833. [Crossref]
- Tulla H1, Takala J, Alhava E, Huttunen H, Kari A, et al. (1991) Respiratory changes after open-heart surgery. Intensive Care Med 17: 365-369. [Crossref]
- Worth H, Schwalen A (1990) [Diagnosis, prevention and therapy of pulmonary complications in heart surgery interventions]. Z Kardiol 79 Suppl 4: 31-38. [Crossref]
- Magnusson L1, Zemgulis V, Wicky S, Tydén H, Thelin S, et al. (1997) Atelectasis is a major cause of hypoxemia and shunt after cardiopulmonary bypass: an experimental study. Anesthesiology 87: 1153-1163. [Crossref]
- Rolla G1, Fogliati P, Bucca C, Brussino L, Di Rosa E, et al. (1994) Effect of pleurotomy on pulmonary function after coronary artery bypass grafting with internal mammary artery. Respir Med 88: 417-420. [Crossref]
- Hachenberg T1, Brüssel T, Roos N, Lenzen H, Möllhoff T, et al. (1992) Gas exchange impairment and pulmonary densities after cardiac surgery. Acta Anaesthesiol Scand 36: 800-805. [Crossref]
- Hachenberg T1, Tenling A, Hansson HE, Tydén H, Hedenstierna G (1997) The ventilation-perfusion relation and gas exchange in mitral valve disease and coronary artery disease. Implications for anesthesia, extracorporeal circulation, and cardiac surgery. Anesthesiology 86: 809-817. [Crossref]
- Tschernko EM, Bambazek A, Wisser W, Partik B, Jantsch U, et al. (2002) Intrapulmonary shunt after cardiopulmonary bypass: the use of vital capacity maneuvers versus off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 124: 732-738. [Crossref]
- Kochamba GS1, Yun KL, Pfeffer TA, Sintek CF, Khonsari S (2000) Pulmonary abnormalities after coronary arterial bypass grafting operation: cardiopulmonary bypass versus mechanical stabilization. Ann Thorac Surg 69: 1466-1470. [Crossref]
- Bender KA1, Alexander JA, Enos JM, Skimming JW (1997) Effects of inhaled nitric oxide in patients with hypoxemia and pulmonary hypertension after cardiac surgery. Am J Crit Care 6: 127-131. [Crossref]
- Costa Leme A, Hajjar LA, Volpe MS, Fukushima JT, De Santis Santiago RR, et al. (2017) Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. JAMA 317: 1422-1432. [Crossref]
- Matte P1, Jacquet L, Van Dyck M, Goenen M (2000) Effects of conventional physiotherapy, continuous positive airway pressure and non-invasive ventilatory support with bilevel positive airway pressure after coronary artery bypass grafting. Acta Anaesthesiol Scand 44: 75-81. [Crossref]
- Norregaard O, Jensen TM, Vindelev P (1996) Effects of inspiratory pressure support on oxygenation and central haemodynamics in the normal heart during the postoperative period. Respir Med 90: 415-417.
- Overend TJ1, Anderson CM, Lucy SD, Bhatia C, Jonsson BI, et al. (2001) The effect of incentive spirometry on postoperative pulmonary complications: a systematic review. Chest 120: 971-978. [Crossref]
- Stock MC, Downs JB, Gauer PK, Alster JM, Imrey PB (1985) Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest 87: 151-157. [Crossref]
- Thomas JA, McIntosh JM (1994) Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Phys Ther 74: 3-10; discussion -6. [Crossref]
- Weindler J1, Kiefer RT (2001) The efficacy of postoperative incentive spirometry is influenced by the device-specific imposed work of breathing. Chest 119: 1858-1864. [Crossref]
- Marvel SL, Elliott CG, Tocino I, Greenway LW, Metcalf SM, et al. (1986) Positive end-expiratory pressure following coronary artery bypass grafting. Chest 90: 537-541. [Crossref]
- Minkovich L, Djaiani G, Katznelson R, Day F, Fedorko L, et al. (2007) Effects of alveolar recruitment on arterial oxygenation in patients after cardiac surgery: a prospective, randomized, controlled clinical trial. J Cardiothorac Vasc Anesth. 21: 375-378. [Crossref]
- Murphy GS, Szokol JW, Curran RD, Votapka TV, Vender JS. (20011) Influence of a vital capacity maneuver on pulmonary gas exchange after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 5: 336-340. [Crossref]
- Rothen HU1, Sporre B, Engberg G, Wegenius G, Reber A, et al. (1995) Prevention of atelectasis during general anaesthesia. Lancet 345: 1387-1391. [Crossref]
- Rothen F, Pieranski P (1996) Mechanical equilibrium of conformal crystals. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 53: 2828-2842. [Crossref]
- Edmunds LH AJ (1980) Effects of cardiopulmonary bypass on the lungs. In: AP F, editor. Pulmonary disease ans disorders. New York Mc Graw-Hill. p. 1733.
- Matuschak GM (1997) Pulmonary dysfunction after surgery involving cardiopulmonary bypass: do we understand the mechanisms? Crit Care Med 25: 1778-1780. [Crossref]
- Rothen HU1, Sporre B, Engberg G, Wegenius G, Högman M, et al. (1995) Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology 82: 832-842. [Crossref]
- Rothen HU1, Neumann P, Berglund JE, Valtysson J, Magnusson A, et al. (1999) Dynamics of re-expansion of atelectasis during general anaesthesia. Br J Anaesth 82: 551-556. [Crossref]
- Hajjar L, Galas F, Nozawa E, Leme A, Shigemi C, et al. (2008) Effects of alveolar recruitment in patients after cardiac surgery: a prospective, randomized, controlled clinical trial. Critical Care 12(Suppl 2): 314.
- Pasquina P, Merlani P, Granier JM, Ricou B (2004) Continuous positive airway pressure versus noninvasive pressure support ventilation to treat atelectasis after cardiac surgery. Anesth Analg 99: 1001-1008, table of contents. [Crossref]
- Karim N, Yazigi A, Sleilaty G, El Asmar B, Haddad F (2005) Effects of repeated vital capacity maneuvers in ICU after coronary surgery. Anesthesiology 103: A1405.
- Wynne R, Botti M (2004) Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: clinical significance and implications for practice. Am J Crit Care 13: 384-393. [Crossref]
- Berry CB1, Butler PJ, Myles PS (1993) Lung management during cardiopulmonary bypass: is continuous positive airways pressure beneficial? Br J Anaesth 71: 864-868. [Crossref]
- Hachenberg T1, Tenling A, Rothen HU, Nyström SO, Tyden H, et al. (1993) Thoracic intravascular and extravascular fluid volumes in cardiac surgical patients. Anesthesiology 79: 976-984. [Crossref]
- Thorvaldson J, Ilebekk A, Leraand S, Kiil F (1984) Determinants of pulmonary blood volume. Effects of acute changes in pulmonary vascular pressures and flow. Acta Physiol Scand 121: 45-56. [Crossref]
- Byrick RJ, Kay C, Noble WH (1977) Extravascular lung water accumulation in patients following coronary artery surgery. Can Anaesth Soc J 24: 332-345. [Crossref]
- Louagie Y1, Gonzalez E, Jamart J, Bulliard G, Schoevaerdts JC (1993) Postcardiopulmonary bypass lung edema. A preventable complication? Chest 103: 86-95. [Crossref]



