Amorphous micro- and nano- sphere of CuSO4 were synthesized on silicon or glass surface by evaporating ethanol from a CuSO4 solution in ethanol. Factors, such as substrate and concentration, on the synthesis of the nano/microspheres were studied. The phenomenon was not observed from solutions of other ethanol soluble salts, such as CuCl2, Cu(NO3)2, Cu(OAc)2, FeCl2, FeCl3, NiCl2, CoCl2, NiSO4.
Nanospheres and microspheres have been widely used in fields such as agriculture, medicine, manufacturing, environment, energy, and cosmetic industries. These micro- and nano- materials can be synthesized from a variety of methods including hydrothermal synthesis [1] attrition [2] pyrolysis [3] thermal plasma [4] inert-gas condensation [5] sol gel process [6] etc. The synthesized materials include metals, polymers, metal oxides, ceramics, and composites. However, few works demonstrated the formation of micro- or nano- spheres from water-soluble salt [7] When salts are recrystallized in solvents, the salts typically precipitated out in the form of regular or irregular crystals, which is a common property of salts. In this short communication, we demonstrate the formation of amorphous micro- and nano- sphere of CuSO4 on silicon or glass surface by evaporating ethanol from a CuSO4 solution in ethanol.
In our approach, a substrate (glass, silicon, or gold coated silicon plate) was submerged in a 20-mL vial with a 0.5 mL CuSO4 solution in ethanol in it. Ethanol was allowed to evaporate at ambient temperature. The precipitated CuSO4 solid on the substrate adopted a spherical shape at nanometer or micrometer dimension (Figure 1), which is a typical character of amorphous particles because of their microstructural isotropy. The amorphous character is also evidenced by the appearance of pimple, raspberry-like surface, in contrary to anisotropic crystalline whiskers corresponding to crystals. Some of the incomplete particles are in the shape of puckered plate, or hemispheres with defect.
The micro- and nano-spheres were only observed when ethanol was used. When preparing in DMF and water, irregular cluster of particles was observed from DMF or aqueous solutions.
Surface property of substrates also affects the formation of spheres, as evidenced by the observation of spheres on silicon or glass surfaces, but not on gold surface , suggesting the surface property plays a critical role in the formation of spheres. It is likely that the OH groups on silicon (there is a naturally oxidized thin film of Si on silicon) or glass surfaces provided multiple nucleation sites for Cu to precipitate out of the solution, which assisted the formation of the spheres.
When the concentration of CuSO4 is lower than 1×10-4 M, the irregular particles are small and not complete in the sphere shape (Figure 4a). A large quantity of the nano-/micro- spheres were found when the CuSO4 concentration were between 1×10-4 M and 1×10-3 M (Figure 4b-e). When the CuSO4 concentration was higher than 4×10-3 M, larger crystals were formed (Figure 4f).
Under similar conditions, only micro- and nano- spheres of were observed. Such phenomenon was not observed from solutions of other ethanol soluble salts, such as . However, we cannot rule out the possibility to grow amorphous spheres from these salt solutions by varying the solvent, substrates, temperature, concentration etc., which is under investigation in our lab now. The property of the nano/microspheres due to their large surface is under investigation as well. Although it is not sure whether the growth of nano- and micro- spheres is a unique phenomenon of CuSO4 in ethanol, the discovery of the micro/nano- spheres of a salt solution may lead to the development of salt based nano/microspheres and applications of these nano/microspheres.
In conclusion, we have synthesized nano/microspheres of by using a facile solvent evaporation method. These spheres may have applications in medical and industrial fields. It is a hypothesis that the formation of amorphous nano/microspheres may be due to relatively faster evaporation of ethanol than other solvents such as water, which resulted in differential stress and distribution of porosity of the precipitated particles . However, lack of mechanism study in the past makes it difficult to explain the phenomenon that only nano/microspheres of but not other salts, were observed. Further study on the growth of amorphous spheres from other salt solutions is critical for the mechanism study in the future.

Figure 1. Scanning electron microscopy (SEM) images of CuSO4 micro- and nano- spheres grown on silicon substrates. The samples were prepared by submerging a silicon substrate in a 0.5 mL of 0.4 mM (left) and 3 mM of CuSO4 (right) in ethanol solutions and ethanol was allowed to evaporate at ambient condition.
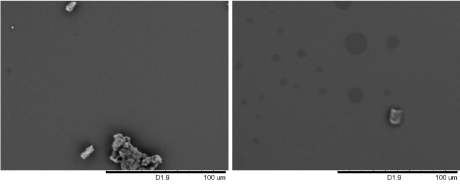
Figure 2. Scanning electron microscopy (SEM) images of CuSO4 structures grown on silicon substrates. The samples were prepared by submerging a silicon substrate in a 0.5 mL of 1 mM of CuSO4 in DMF (left) and aqueous solutions (right), and the solvents were allowed to evaporate at ambient condition.
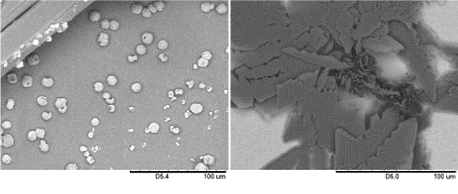
Figure 3. SEM of CuSO4 structures grown on glass and gold substrates. The samples were prepared by submerging a glass (left) and gold (right) substrate in a 0.5 mL of 2 mM of CuSO4 in ethanol, and the solvents were allowed to evaporate at ambient condition.

Figure 4. SEM images of CuSO4 micro- and nano- spheres grown on glass substrates. The samples were prepared by submerging a glass substrate in a 0.5 mL of CuSO4 solutions in ethanol and ethanol was allowed to evaporate at ambient condition.
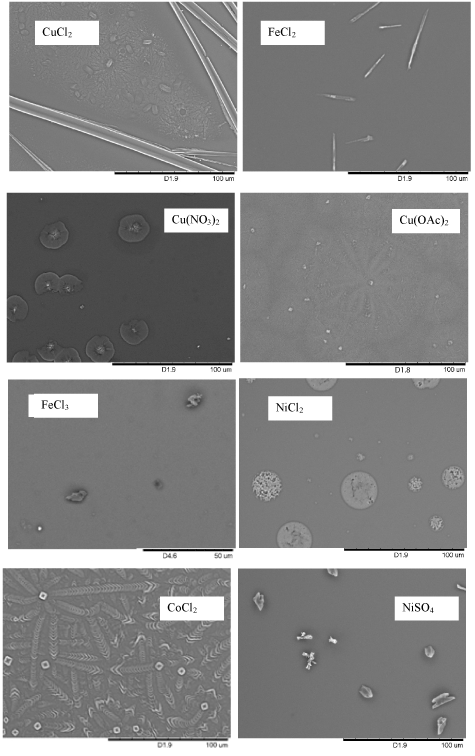
Figure 5. Scanning electron microscopy (SEM) images of structures of various salts grown on silicon substrates. The samples were prepared by submerging a silicon substrate in a 0.5 mL of 3 mM salt solutions in ethanol and ethanol was allowed to evaporate at ambient condition.
2021 Copyright OAT. All rights reserv
- Zheng M, Liu Y, Xiao Y, Zhu Y, Guan Q, et al. (2009) An Easy Catalyst-Free Hydrothermal Method to Prepare Monodisperse Carbon Microspheres on a Large Scale. J Phys Chem C 113: 8455-8459.
- Charitidis C, Georgiou P, Koklioti M, Trompeta A, Markakis V (2014) Manufacturing nanomaterials: from research to industry. Manufacturing Rev 1: 11.
- Yang W, Yang W, Mao S, Yang J, Shang T, et al. (2016) Large-deformation and high-strength amorphous porous carbon nanospheres. Sci Rep 6: 24187.
- Nguyen T, Tran T (2014) Multicomponent nanoarchitectures for the design of optical sensing and diagnostic tools. RSC Adv 4: 916-942.
- Lee K, Juhng W, Choi B (2006) Effect of variables in inert gas condensation processing on nanoparticle trajectory simulated by finite volume method. J Nanosci Nanotechnol 6: 3433-3437. [Crossref]
- Hench L, West J (1990) The sol-gel process. Chem Rev 90: 33-72.
- Jian G, Feng J, Jacob RJ, Egan GC, Zachariah MR (2013) Super-reactive nanoenergetic gas generators based on periodate salts. Angew Chem Int Ed Engl 52: 9743-9746. [Crossref]
- Evans A, Davidge R (1970) The strength and oxidation of reaction-sintered silicon nitride. J Mat Sci 5: 314-325.
- Lange F, Metcalf M (1983) Processing-Related Fracture Origins: II, Agglomerate Motion and Cracklike Internal Surfaces Caused by Differential Sintering. J Am Ceram Soc 66: 398-406.





