Abstract
Objective: To evaluate the surgical approaches and prognosis of thyroid carcinomas invading the adjacent structures.
Methods: The medical records of 197 patients with a pathology diagnosis of thyroid cancer were retrospectively reviewed.
Results: 17 patients (9%) with thyroid carcinoma invading surrounding structures were included. Patients were initially divided into 2 groups on the basis of tumor histology: papillary (Group A) and nonpapillary (Group B). Then patients were divided into 3 groups: Group 1: patients who underwent total thyroidectomy. Group 2: patients who underwent total thyroidectomy with shaving resection. Group 3: patients who underwent total thyroidectomy with extensive surgery. All patients who didn’t survive were more than 45-year-old. The survival rate was better in group A compared to group B (92% versus 20%). The survival rate increased from Group 1 through Group 3.
Conclusion: Age and histologic type are important in determining the prognosis of locally invading thyroid cancer.
Key words
thyroid cancer, invasion, age, surgery, prognosis
Introduction
Extracapsular spread is seen in approximately 5 to 15% of cases of well-differentiated thyroid carcinoma. The predominant pathology leading to invasive spread is papillary thyroid carcinoma. Locally advanced thyroid cancer is secondary to direct primary tumor extension or extracapsular extension of involved lymph nodes (1-15). Patients with extrathyroid extension have an increased incidence of local recurrence, regional spread, and distant metastasis. The most common structures involved by extrathyroid extension in the central compartment are strap muscles, recurrent laryngeal nerve (RLN), trachea, laryngeal framework, esophagus, and pharyngeal constrictors. Structures in the lateral neck compartment that can be involved include the carotid artery, internal jugular vein, vagus nerve, spinal accessory nerve, and phrenic nerve [1].
Surgical resection is the primary treatment for patients with locally aggressive thyroid cancer. The principles of surgical management of locally advanced thyroid cancer are the following: [1] removal of all gross tumor, [2] preservation of functioning structures, [3] preservation of vital structures, and [4] use of adjuvant therapies. Some controversies still persist between authors advocating conservative surgical management since they did not find any improved local control and survival with the aggressive approach of invasive well differentiated thyroid carcinoma, and those favouring aggressive surgical approach over shave resection, reporting better local control and survival with extensive surgery [16-35].
The aim of this study was to review our experience in the surgical management of locally invading nonanaplastic thyroid cancers.
Methods
Data retrieval
We retrospectively reviewed the medical records of all patients with a diagnosis of thyroid cancer treated at Hotel Dieu de France Hospital, Lebanon from 1997 through 2007. These patients were identified using the results of their postoperative pathology specimens. A total of 197 cases were included. Of the 197 sample, 17(9%) patients were identified having invasion of at least one adjacent structure. The 17 patients were divided initially into 2 groups according to the presence or absence of papillary carcinoma (as per postoperative pathology results): group A consisted of 12 patients with papillary carcinoma and group B consisted of the remaining 5 patients with nonpapillary carcinomas. Then these 17 patients were divided again into 3 groups according to the type of surgery performed: Group 1: patients who underwent total thyroidectomy or tumor ablation with no « shaving » or extensive resection. Group 2: patients who underwent a total thyroidectomy or tumor ablation with « shaving » resection. Group 3: patients who underwent total thyroidectomy or tumor ablation with extensive surgery.
Inclusion & exclusion criterias
All the relevant clinical variables were analyzed including clinical presentation, pre and postoperative tumor histology, prior medical or surgical history of thyroid disease. All patients with locally invasive primary or recurrent nonanaplastic thyroid carcinomas with or without distant metastasis, who were treated at our department between 1997 and 2007 were included. Patients who were preoperatively diagnosed with anaplastic thyroid carcinoma on pathology were excluded. Invasion was assessed either preoperatively clinically, radiologically and macroscopically (by laryngoscopy) or postoperatively by pathologic examination.
Surgical management
Preoperative ultrasound and fine-needle aspiration were done on all patients. Tumor extension was assessed preoperatively either by a computed tomography (CT) (Figure 1) or by a magnetic resonance imaging (MRI) scan. Furthermore, all patients underwent a preoperative direct laryngoscopy. All surgeries were done under general anesthesia using horizontal cervical incision. Only one sternotomy was necessary for mediastinal lymph node dissection. The type of resection was chosen in the operating room depending on either the presence of macroscopic intraoperative findings of invasion of adjacent structures or on the result of the frozen section. The thyroidectomies were all performed in a similar fashion with careful dissection attempting to identify and preserve the important adjacent structures such as the parathyroid glands with their vascular supply, as well as the recurrent laryngeal nerves. Inadvertently removed or unequivocally devascularized parathyroid glands were removed routinely for immediate auto transplantation.
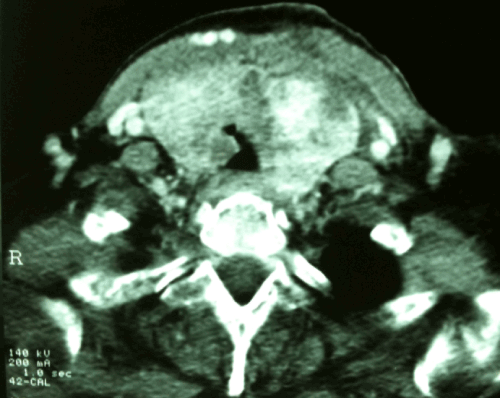
Figure 1. CT Scan of the neck showed bilateral thyroid tumor with right intra luminal tracheal invasion.
Statistical analysis
Quantitative data are presented as mean ± standard deviation. Qualitative data are reported as proportions. Survival curves were built using the Kaplan-Meier method and the survival rates were compared using the log-rank test. All statistical computings were performed using the SPSS® software (SPSS, Inc., Chicago, Ill.).
Results
17 patients with a preoperative diagnosis of nonanaplastic thyroid cancer with invasion of at least one adjacent structure satisfying inclusion/exclusion criteria were included in the study. The series included 12 women (71%) and 5 men (29%). The mean age at diagnosis was 53 years (extreme: 15 - 82 years). The symptoms of upper aerodigestive tract invasion were hemoptysis, stridor, hoarseness, dysphagia and aspiration. Only one patient had distant metastasis at the time of diagnosis.
The most common sites of involvement were the overlying striated muscles (83%), recurrent laryngeal nerves (31%), larynx (35%), trachea (29%) followed by esophagus (17%). The initial staging of the patients was T3-N0-M0, T3-N1-M0, T4a-N0-M0, T4a-N1-M0, T4a-N0-M1.
Total thyroidectomy was the surgical procedure performed on all our patients except on those who had recurrent disease following initial resection and who then underwent resection of their recurrent cancer. Two patients had recurrent disease following thyroid resection for noninvasive carcinoma; one case of follicular carcinoma which was treated at first with right lobectomy and then with total thyroidectomy and one case of medullary carcinoma which was treated 5 months earlier with total thyroidectomy with bilateral lymph node dissection. These 2 patients were not operated on in our institution. Lymph node dissection of the central compartment (level VI) and of the ipsilateral jugulocarotid chains (levels III and IV) was performed on all patients treated with total thyroidectomy. Two patients underwent bilateral lymph node dissection of the jugulocarotid chains because of the involvement of both lobes of the thyroid gland by the cancer. Palpable lymph nodes or lymph nodes that were found highly suspicious for metastasis on intraoperative findings outside the jugulocarotid chains were also dissected. Thus a modified lymph node dissection was achieved in 4 patients.
Local resection of all macroscopic invaded structures was performed on all patients. Thus, the extension of local resection of the tumor depended on the macroscopic extension seen intraoperatively. When the tumor was adhering to either laryngo-trachea or esophagus without signs of invasion, a “shaving” resection was performed. When the trachea, the esophagus or the larynx were invaded by the tumor, partial resection of the invaded structure was performed. This is what we call extensive surgery or extensive resection. Postoperatively, oral L thyroxine and adjuvant radioactive iodide were given to all patients and 15 patients repectively. During follow-up, five patients died and all were more than 45-year-old. None of the patients underwent postoperative external radiation therapy.
Comparision of groups A and B
On final pathology, 12 patients had papillary carcinomas (Group A) of the thyroid (70%), 2 patients had medullary carcinomas (12%), one patient had follicular carcinoma (6%) and 2 patients had follicular and undifferentiated thyroid carcinomas (12%) (Group B).
Table 1 compares invasion symptoms, type of surgery, invaded structures and recurrence rates between the groups A and B. In group A, 4 patients had hoarsness (33%), 3 patients had dysphagia (25%), 2 patients had aspiration (17%), one patient had hemoptysis (8%) and one had stridor (8%). In group B, 2 patients had hoarsness (40%), one patient had stridor (20%), one patient had dysphagia (20%), and none had aspiration or hemoptysis (0%). Invasion symptoms of adjacent structures was equally distributed in the 2 groups A and B.
Table 1. Comparison between Group A (patients with invading papillary cancer) and Group B (patients with invading nonpapillary cancer)
|
|
Group A n (%) |
Group B n (%) |
Invasion
symptoms
of
adjacent structures |
Existence |
6 (50%) |
2 (40%) |
Dysphonia |
4 (33%) |
2 (40%) |
Stridor |
1 (8%) |
1 (20%) |
Dysphagia |
3 (25%) |
1 (20%) |
Aspiration |
2 (17%) |
0 (0%) |
Hemoptysis |
1 (8%) |
0 (0%) |
Type
of
surgery |
Simple thyroidectomy |
2 (17%) |
1 (20%) |
Shaving |
4 (33%) |
3 (60%) |
Extensive surgery |
6 (50%) |
1 (20%) |
Structures
invaded |
Striated muscles |
9 (75%) |
5 (100%) |
Recurrent nerve |
4 (37%) |
1 (20%) |
Larynx |
4 (33%) |
2 (40%) |
Trachea |
4 (33%) |
1 (20%) |
Esophagus |
1 (8%) |
2 (40%) |
Recurrence |
3 (25%) |
3 (60%) |
The striated muscles were invaded in 9 patients (75%) in group A and in all patients (100%) in group B. Four patients in group A (36%) and one patient in group B (20%) had recurrent nerve invasion. Recurrent nerve was sacrificed in 3 cases. Two have had surgical resection of papillary thyroid carcinomas with microscopic residual tumor tissue left around the recurrent laryngeal nerve to preserve it (provided that this residual tissue will be controlled by adjuvant 131I). The larynx was invaded in 4 patients in group A (33%) and in 2 patients in group B (40%). Four patients in group A (33%) and one patient in group B (20%) had invasion of the trachea. The esophagus was invaded in one patient in group A (8%) and in 2 patients in group B (40%).
Simple thyroidectomy was performed on 3 patients in group A (25%). Four patients in group A (33%) and 3 patients in group B (60%) had undergone shaving technique. Extensive surgery (n = 7) (wedge resection of the trachea n=2, partial laryngectomy n =3, and resection-anastomosis of the trachea n= 1) was performed on 5 patients (42%) in group A and 2 patients (40%) in group B. Concerning the type of surgery performed, no difference was noted for simple thyroidectomy in the group of patients with papillary cancer (Group A) when compared to the group of patients with nonpapillary cancer (Group B) where simple thyroidectomy was not sufficient (0). Also, there was no significant difference regarding extensive surgery between the 2 groups which means that extensive surgery was performed with equal frequency in the group of patients with papillary cancer and in the group of patients with nonpapillary cancer.
During the follow-up (0 - 146 months), of all patients having papillary carcinoma (group A), one patient died from liver and lungs metastasis at 11 months following surgical treatment. The 5 years’ survival rate in group A was 92%. Conversely, of the 5 patients in group B, only one patient (20%) survived the 5-year follow-up. The major etiology of death in this group was the cancer itself, either via recurrent disease with distant metastasis (2 patients), follicular cancer with undifferentiated component (1 patient), medullary carcinoma (1 patient) or fatal local recurrence of a follicular carcinoma without distant metastasis (1 patient). One patient died due to insufficient surgical control of the undifferentiated component. The survival curves of groups A and B are shown in Figure 2, showing a statistical difference in survival favoring the group A (p= 0.01). Recurrence was seen in 3 patients in group A (25%) and in 3 patients in group B (60%). An observed difference in the recurrence rate was noted between group A and group B.
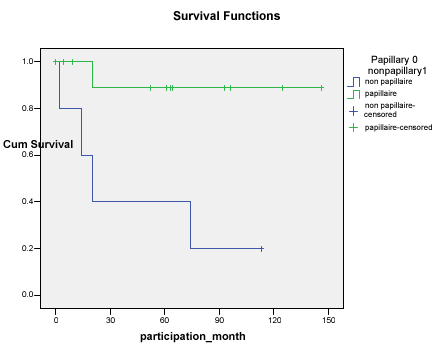
Figure 2. Survival of patients with invasive papillary carcinoma versus invasive nonpapillary carcinoma.
Comparison of groups 1, 2, and 3
The influence of the presence and the type of invasion symptoms on the recurrence rates between groups 1, 2 and 3 is shown in Table 2. No significant differences were found between the 3 groups. However, these symptoms were more frequently encountered in those patients who had extensive surgery (80%) compared to patients who have had « shaving » or simple thyroidectomy.
Table 2. Comparison between patients with locally invasive thyroid cancer treated with different types of surgery.
|
|
Group 1 n (%) |
Group 2 n (%) |
Group 3 n (%) |
Invasion
symptoms
of
adjacent
structures |
Existence |
2 (50%) |
1 (14%) |
4 (67%) |
Dysphonia |
0 (0%) |
1 (14%) |
5 (72%) |
Stridor |
0 (0%) |
1 (14%) |
1 (14%) |
Dysphagia |
1 (33%) |
0 (0%) |
3 (43%) |
Aspiration |
0 (0%) |
0 (0%) |
2 (29%) |
Hemoptysis |
0 (0%) |
0 (0%) |
1 (14%) |
Recurrence |
0 (0%) |
2 (29%) |
4 (57%) |
The 5-year survival rate in the non extensive surgery group was 100%. In fact, 2 of the 7 patients died from local recurrence and distant metastasis following initial « shaving ». All the patients who underwent simple thyroidectomy and 5 of the 7 patients who underwent « shaving » resection survived till the end of the study (survival rates at 5 years were 100% and 72% respectively). The 5-year survival rate in group 3 was 57%. Three of the 7 patients died; 2 of them died as a consequence of the undifferentiated component of their follicular cancer that was locally very aggressive and had locally recurred. The survival curves for the extensive and non-extensive surgery groups (Figure 3) were not statistically divergent (p = 0.886). The postoperative recurrence rates differed between the simple thyroidectomy (0%) and the extensive surgery cases (57%).
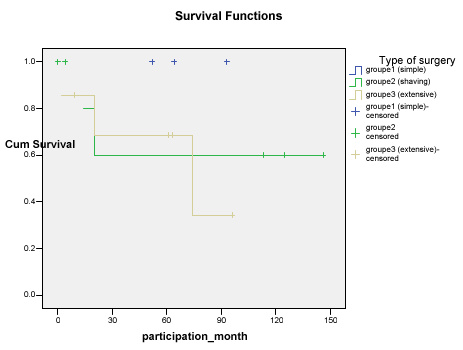
Figure 3. Survival curves of patients with invasive WDTC undergoing extensive versus nonextensive surgery.
Discussion
There was a statistically significant difference in the survival rates between patients with papillary thyroid carcinoma (Group A) and those with nonpapillary cancer (Group B) in our series (92% versus 20%) p=0.011. In fact, this was noted by Ballantyne [27] who reported that the survival rates were low for patients who had tumors other than papillary or papillary and follicular carcinomas. Bayles et al. [18] compared survival on basis of the histologic type of thyroid cancer (differentiated versus undifferentiated) and reported a better survival in the well-differentiated group.
Our results did not show a statistically significant difference in survival between extensive surgery and shave excision. Thus, they do not show an advantage of aggressive treatment over conservative treatment on the survival rates. Moreover, the survival rates were better in the non extensive surgery group (without reaching statistical significance). Survival rates at 5 years in our series were 100% in groups 1, 72% and 57% in group 2, and 3 respectively. All patients who didn’t survive after the procedure were more than 45 year-old. In some retrospective studies [1,5,19,20,25], laryngotracheal invasion was noted to be an independent prognostic factor for survival. Shaving is acceptable if there is cartilage invasion but no direct intraluminal involvement [16-25]. This approach has been shown in many studies to be as effective in locoregional control and survival as complete resection, without the morbidity that affects swallowing, speech, and voice. Residual microscopic disease can be effectively controlled with adjuvant radioiodine ablation or external radiotherapy [1,5]. Shave excision is not appropriate in cases of direct intraluminal invasion, which can lead to death. Complete resection is necessary in these cases. When all gross tumor was removed from the primary site, shave excision and complete resection techniques had similar survival rates. Some authors proposed and advocated shave resection for tumors with minimal invasion; for patients with gross intraluminal spread, techniques enabling complete resection of tumor were advocated [11-25]. In a systematic review, Kim et al. [5] reported that the « shaving » group and resection group had varying survival rates between 55 and 100% and between 38% and 100% respectively [16-35].
The rates of recurrences were very similar between the shave and laryngotracheal resection groups (15% to 28% for shaving and 7% to 39% for resection) (16-35). In our series, recurrence rates were higher in patients treated with extensive resection than in patients treated with conservative approach (0% in group 1, 29% in group 2 and 57% in group 3). Of note is that patients treated with extensive resection had a deeper invasion of the surrounding structures, but this difference was not statistically significant. Thus, in the present investigation even the recurrence rates were not different from previously reported values regarding the practice of « shaving » technique.
The treatment of the recurrent laryngeal nerve invasion generates considerable controversy. Appropriate preoperative evaluation of the vocal cords is imperative. If the recurrent laryngeal nerve is paralyzed preoperatively, it obviously needs to be sacrificed. If the nerve is functioning, however, the decision to sacrifice the nerve becomes difficult. If preservation of the nerve requires leaving gross tumor behind, then the nerve should be sacrificed. Before one recurrent laryngeal nerve is sacrificed, however, it is very important to make sure that the opposite nerve is not directly involved with the tumor and that it can be preserved. If preservation of the nerve requires leaving microscopic disease, then the nerve should be spared. Leaving microscopic disease on the recurrent laryngeal nerve does not lead to decreased survival or increased recurrence, in comparison with resection of the nerve [1,2,19,20]. Patients with residual microscopic disease should have postoperative radioiodine ablation and possibly external radiotherapy. For some authors [19,20], no survival benefit was realized with nerve sacrifice.
Appropriate preoperative imaging with CT or MRI, cervical ultrasound, and bronchoscopy is also very essential [1,12,15]. Invasion of the esophagus and pharynx is usually confined to the muscularis layer, without extension into the submucosa or mucosa. Invasion of the muscularis layer often leads to compressive dysphagia symptoms. Excision of the muscularis layer can be performed to obtain negative margins. With full-thickness or circumferential involvement of the cervical esophagus, a segmental resection is necessary.
The role of therapeutic neck dissection in the setting of thyroid carcinoma, typically including levels II through V, is accepted when clinical or radiographic evidence of regional metastasis exists. Elective lateral neck dissection beyond central compartment dissection is somewhat controversial. Some authors [36-38] have advocated bilateral modified radical neck dissections at the time of thyroidectomy in patients with invasive disease because of the risk of nodal recurrence and the impact it may have on distant metastasis and survival.
Adjuvant treatment after complete resection with l-thyroxine suppression and radioactive iodine ablation are considered the treatment of choice for invasive thyroid carcinoma. In cases where tumors are not radioiodine avid and/or the procedure required to obtain complete removal is unacceptable external radiotherapy can be effective in stabilizing locoregional disease with reduction in thyroglobulin levels in some cases [1,5,39]. Some authors routinely using intensity modulated radiation therapy (IMRT) in these patients to offer an effective radiation dose to the thyroid gland and avoid spinal cord injury. Several chemotherapeutic agents have been studied in patients with locally advanced thyroid cancer, but no significant response has been noted. Other agents such as histone deacetylase inhibitors and peroxisome proliferator-activated receptor-g agonists are being studied. These patients require very close follow-up. Thyroglobulin levels, radioiodine scanning, and PET scans should be included in the algorithm for long-term follow-up of patients with locally advanced thyroid carcinoma [1].
In the literature, patients with isolated local and nodal recurrence after conservative treatment were treated with salvage therapy consisting of radical resection, lymph node dissection, and radioactive iodine ablation. Anaplastic transformation was encountered in 14% with a history of incomplete resection in one series [9]. Five-year disease free survival was accomplished in all patients [21] and 100% survival rate in a series of patients younger than 45 years [9]. All patients who didn’t survive in our series were more than 45 year-old. Also of note is that Ballantyne [27], in reviewing the MD Anderson experience, despite the majority of patients having undergone prior treatment, 5-year survival was more than 50% overall and was noted to be more than 70% for patients with papillary or follicular carcinomas.
The current series has several limitations. The small number of patients is to be considered when interpreting the results, mainly acting by under powering the statistical tests and inflating the type II error. The retrospective collect of data is another shortcoming. Selection biases are also inherent to this study, since the patients were selected from the database of tertiary university hospital.
Conclusions
On the basis of our results, we believe that age and histologic type are important in determining the prognosis of locally invading thyroid cancer. Thyroidectomy associated with resection of a major portion of the adjacent structures are compatible with life without guarantying high survival rates.
References
- Patel KN, Shaha AR (2005) Locally advanced thyroid cancer. Curr Opin Otolaryngol Head Neck Surg 13: 112-116. [Crossref]
- McCaffrey JC (2006) Aerodigestive tract invasion by well-differentiated thyroid carcinoma: diagnosis, management, prognosis, and biology. Laryngoscope 116: 1-11. [Crossref]
- Friedman M (1990) Surgical management of thyroid carcinoma with laryngotracheal invasion. Otolaryngol Clin North Am 23: 495-507. [Crossref]
- Talpos GB (1999) Tracheal and laryngeal resections for differentiated thyroid cancer. Am Surg 65: 754-759. [Crossref]
- Kim AW, Maxhimer JB, Quiros RM, Weber K, Prinz RA (2005) Surgical management of well-differentiated thyroid cancer locally invasive to the respiratory tract. J Am Coll Surg 201: 619-627. [Crossref]
- Friedman M, Danielzadeh JA, Caldarelli DD (1994) Treatment of patients with carcinoma of the thyroid invading the airway. Arch Otolaryngol Head Neck Surg 120: 1377-1381. [Crossref]
- Tsumori T, Nakao K, Miyata M, Izukura M, Monden Y, et al. (1985) Clinicopathologic study of thyroid carcinoma infiltrating the trachea. Cancer 56: 2843-2848. [Crossref]
- Britto E, Shah S, Parikh DM, Rao RS (1990) Laryngotracheal invasion by well-differentiated thyroid cancer: diagnosis and management. J Surg Oncol 44: 25-31. [Crossref]
- Tanaka K, Sonoo H, Yamamoto Y, Udagawa K, Arime I, et al. (1999) Analyses of the outcome of locally invasive papillary thyroid carcinomas. Thyroid 9: 1017-1022. [Crossref]
- Martins AS, Melo GM, Valério JB, Langner E, Lage HT, et al. (2001) Treatment of locally aggressive well-differentiated thyroid cancer. Int Surg 86: 213-219. [Crossref]
- Lipton RJ, McCaffrey TV, van Heerden JA (1987) Surgical treatment of invasion of the upper aerodigestive tract by well-differentiated thyroid carcinoma. Am J Surg 154: 363-367. [Crossref]
- Yamamura N, Fukushima S, Nakao K, Nakahara M, Kurozumi K, et al. (2002) Relation between ultrasonographic and histologic findings of tracheal invasion by differentiated thyroid cancer. World J Surg 26: 1071-1073. [Crossref]
- Wein RO (2005) Management of the locally aggressive thyroid carcinoma. American Journal of Otolaryngology-Head and Neck Medicine and Surgery 26 : 186-192.
- Miyauchi A, Ito Y, Miya A, Higashiyama T, Tomoda C, et al. (2007) Lateral mobilization of the recurrent laryngeal nerve to facilitate tracheal surgery in patients with thyroid cancer invading the trachea near Berry’s ligament. World J Surg 31 : 2081-2084. [Crossref]
- Koike E, Yamashita H, Noguchi S, Yamashita H, Ohshima A, et al. (2001) Bronchoscopic diagnosis of thyroid cancer with laryngotracheal invasion. Arch Surg 136: 1185-1189. [Crossref]
- Park CS, Suh KW, Min JS (1993) Cartilage-shaving procedure for the control of tracheal cartilage invasion by thyroid carcinoma. Head Neck 15: 289-291. [Crossref]
- Shvili Y, Zohar Y, Buller N, Laurian N (1985) Conservative surgical management of invasive differentiated thyroid cancer. J Laryngol Otol 99: 1255-1260. [Crossref]
- Bayles SW, Kingdom TT, Carlson GW (1998) Management of thyroid carcinoma invading the aerodigestive tract. Laryngoscope 108: 1402-1407. [Crossref]
- McCaffrey TV, Lipton RJ (1990) Thyroid carcinoma invading the upper aerodigestive system. Laryngoscope 100: 824-830. [Crossref]
- McCaffrey TV, Bergstralh EJ, Hay ID (1994) Locally invasive papillary thyroid carcinoma: 1940-1990. Head Neck 16: 165-172. [Crossref]
- McCarty TM, Kuhn JA, Williams WL Jr, Ellenhorn JD, O'Brien JC, et al. (1997) Surgical management of thyroid cancer invading the airway. Ann Surg Oncol 4: 403-408. [Crossref]
- Kowalski LP, Filho JG (2002) Results of the treatment of locally invasive thyroid carcinoma. Head Neck 24: 340-344. [Crossref]
- Kim KH, Sung MW, Chang KH, Kang BS (2000) Therapeutic dilemmas in the management of thyroid cancer with laryngotracheal involvement. Otolaryngol Head Neck Surg 122: 763-767. [Crossref]
- Nishida T, Nakao K, Hamaji M (1997) Differentiated thyroid carcinoma with airway invasion: indication for tracheal resection based on the extent of cancer invasion. J Thorac Cardiovasc Surg 114: 84-92. [Crossref]
- Czaja JM, McCaffrey TV (1997) The surgical management of laryngotracheal invasion by well-differentiated papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 123: 484-490. [Crossref]
- Melliere DJ, Ben Yahia NE, Becquemin JP, Lange F, Boulahdour H (1993) Thyroid Carcinoma with Tracheal or Thyroid Involvement: Limited or Maximal Surgery?. Surgery 113 : 166-172. [Crossref]
- Ballantyne AJ (1994) Resections of the upper aerodigestive tract for locally invasive thyroid cancer. Am J Surg 168: 636-639. [Crossref]
- Ahmed M, Saleem M, Al-Arifi A, Almahfouz A, Mahasin Z, et al. (2002) Obstructive endotracheal lesions of thyroid cancer. J Laryngol Otol 116: 613-621. [Crossref]
- Yang CC, Lee CH, Wang LS, Huang BS, Hsu WH, et al. (2000) Resectional treatment for thyroid cancer with tracheal invasion: a long-term follow-up study. Arch Surg 135: 704-707. [Crossref]
- Grillo HC, Zannini P (1986) Resectional management of airway invasion by thyroid carcinoma. Ann Thorac Surg 42: 287-298. [Crossref]
- Grillo HC, Suen HC, Mathisen DJ, Wain JC (1992) Resectional management of thyroid carcinoma invading the airway. Ann Thorac Surg 54: 3-9. [Crossref]
- Musholt TJ, Musholt PB, Behrend M, Raab R, Scheumann GF, Klempnauer J, et al. (1999) Invasive differentiated thyroid carcinoma: tracheal resection and reconstruction procedures in the hands of endocrine surgeon. Surgery 126: 1078–1088. [Crossref]
- Sywak M, Pasieka JL, McFadden S, Gelfand G, Terrell J, et al. (2003) Functional results and quality of life after tracheal resection for locally invasive thyroid cancer. Am J Surg 185: 462-467. [Crossref]
- Donnelly MJ, Timon CI, McShane DP (1994) The role of total laryngectomy in the management of intraluminal upper airway invasionby well-differentiated thyroid carcinoma. ENTJ 73: 659–662.
- Ozaki O, Sugino K, Mimura T, Ito K (1995) Surgery for patients with thyroid carcinoma invading the trachea: circumferential sleeve resection followed by end-to-end anastomosis. Surgery 117: 268-271. [Crossref]
- Machens A, Hinze R, Lautenschläger C, Thomusch O, Dralle H (2001) Thyroid carcinoma invading the cervicovisceral axis: routes of invasion and clinical implications. Surgery 129: 23-28. [Crossref]
- Machens A, Hinze R, Dralle H (2001) Surgery on the cervicovisceral axis for invasive thyroid cancer. Langenbecks Arch Surg 386: 318-323. [Crossref]
- Cody HS 3rd, Shah JP (1981) Locally invasive, well-differentiated thyroid cancer. 22 years' experience at Memorial Sloan-Kettering Cancer Center. Am J Surg 142: 480-483. [Crossref]
- Farahati J, Reiners C, Stuscheke M, Muller SP, Stuben G, et al. (1996) Differentiated thyroid cancer. Impact of external radiotherapy in patients with perithyroidal infiltration (stage pT4). Cancer 77: 172–180. [Crossref]



