Editorial
Outline
Ischemic cardiomyopathy is the most common type of dilated cardiomyopathy. Its surgical treatment is one of the most challenging topic in cardiac surgery, with a history starting about 50 years ago. Several types of correction have been devised with varying outcome. The problem of mitral regurgitation is still an open issue. Hundreds of studies tried to resolve the dilemma of the best surgical treatment, but they depicted a very spread outcome with several relapses and suboptimal functional improvement, sometimes with unexpected, controversial or unmeaning results.
This variability could be due to the complex anatomo-functional structure of the left ventricle and to the lack of a multifactorial, integrated approach that takes it into account.
The shape of a volume
The two left ventricles in Figure 1 have the same end-systolic volume (105 ml). But a different shape.
The left one is a spherical ventricle while the right one is an elliptical ventricle. Probably, the first one will not recover a normal function also after the correction of underlying disease, while the second one will probably regain a better function.
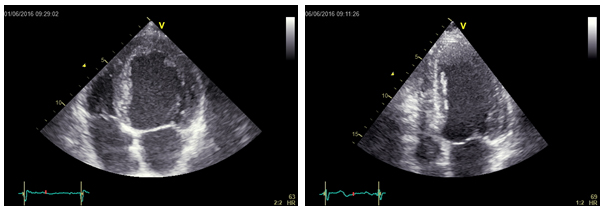
Figure 1. Two different ventricular shapes (and structures) for the same end-systolic volume.
This difference in potential for recovery is basically due to the unfavourable geometry of the spherical ventricular chamber that conditions the disarrangement of fibers’ disposition and a wasteful mechanics. Fibers’ disposition and its direct consequence, left ventricular torsion, is crucial to the normal function of the left ventricle, both in systole and in diastole. Torsion is the functional expression of the normal 3D architecture of the left ventricular wall: it is due to the shortening of obliquely-oriented fibers’ bundles that obtains the opposite twisting of the apex and the base of the ventricle, squeezing the ventricular chamber in a high-efficiency, energy-sparing mechanics [1]. This movement is lost when 3D structure is altered and it is a very sensitive parameter in any pathologic state of the myocardium. Just recently, thanks to the echocardiographic technique of 2D speckle tracking imaging, the visualization and therefore the study of fibers’ disposition was available with a simple, bedside, reproducible technique. This tool rapidly spread the consideration of this peculiar feature in everyday clinical practice. The true grade of ventricular dysfunction is therefore hidden from naked eye: it is linked to the 3D anatomical structure of the myocardium, a feature that, until now, was difficult to study and consider but represents the essential base of normalcy.
The paradigm of normalcy
The definition of the left ventricle has always been difficult, due to its complex, irregular and three-dimensional geometry. If we try to equate the left ventricle to a geometrical figure, the most suitable definition is that of a prolate ellipsoid (three-dimensional analogue of an ellipse). The similarity with an ellipsoid is useful to understand the importance of the two diameters (long and short axis) in the final geometrical (and functional) shape. However, the presence of papillary muscles, the myocardial trabeculation, the presence of the mitral valve and the variable shape of the outflow tract make the left ventricular chamber very different from a plain geometrical figure.
The normal left ventricle is a very complex structure, with a 3D, three-layered interlaced fibers’ disposition; a unique interaction between myocardium and mitral valve; a complex and elastic labyrinth of sarcoplasmic reticulum; a contraction-suitable system for blood perfusion; an electrical conduction system that assures synchronicity, and a contraction-relaxation cycling (systole and diastole) in which both phases are in some way active.
This multifactorial definition of normalcy mirrors on the difficulty in selecting homogeneous series of patients to be included in prospective and randomized studies. For example, a selection criterion like “30% ejection fraction” can include several different shapes and/or ventricular volumes, as “grade three mitral regurgitation” may collect several different tenting areas or angles. And more, both these definitions of severity of disease disregard, for example, the grade of fibers' disarrangement or the grade of irreversible fibrosis of the ventricle.
A selection of patients made by “macroscopic” parameters can hide structural and functional disparities, which can affect the results, jeopardizing the effects of therapies, whether they be medical or surgical.
Left ventricular reconstruction
History of left ventricular reconstruction, from first successful open excision [1] to STICH trial [1], clearly demonstrated that significant volume reduction is essential to obtain a good functional result but it is not the only parameter to rely on. From endoventricular circular patch plasty [1] up to date, several evidences [1-5] showed that left ventricular shape and geometry have at least an equivalent value to restore cardiac function and obtain steady clinical results.
Clinical and functional outcomes after “left ventricular volume reduction surgery” were very variable and generally unsatisfying: redilation of ventricular chamber and the recurrence or new onset of mitral regurgitation affected the medium and long-term results. The main role in this lack in reverse remodelling was played by the unfavourable geometry of the reduced left ventricle that became a box-shaped chamber with the amputation of the apex. This led to an expensive mechanics, displaced fibers’ orientation, further impairment or loss of torsion movement, worsening of diastolic properties and progressive redilation.
In the light of these limited results, many surgeons paid increasing attention to the respect of normal geometry and structure of the heart, demonstrating that better results are obtained when surgery aims to rebuild an elliptic chamber rather than just a smaller one. The new concept of “surgical ventricular reconstruction” (SVR) took the place of “volume reduction” surgery.
To overcome the doubts on effectiveness of this complex surgical procedure an international, randomised clinical trial was set up from 2002 to 2009. The STICH (Surgical Treatment of Ischemic Heart Failure) trial had the great value to be a worldwide study coordinated by the Duke University. Study sites were selected after been certified for their ability in reducing left ventricular volume by at least 30% and the study generated an intense experience’s sharing among participants.
Unfortunately, the percentage of left ventricular volume reduction obtained in CABG+SVR STICH patients was only 19%, that is significantly inferior to the target percentage required in the study protocol (30%). This datum aroused many criticisms [1-5] about the reliability of the conclusions of the study itself, asserting that the anatomical change induced by SVR was not associated with an improvement in symptoms, exercise tolerance or reduction in the rate of death or hospitalization for cardiac causes [2]. Actually, the “anatomical change” obtained missed one of the major endpoints of the study and was not enough to reduce end-systolic ventricular volume under a value (50 ml/m2) known to be a predictor of inverse remodeling [1]. This datum indirectly confirmed the negative outcome reported for large ventricles obtained by different surgical techniques not rebuilding small, elliptical volumes [4,5,8,1], but did not respect the minimum percentage of volume reduction required to the certified Centres.
As a consequence, the debate about need and usefulness of surgical left ventricular reconstruction is far from over still after the STICH trial results [1] and the technique has been localised and reserved to highly skilled Centres of excellence.
Mitral valve repair/replacement
The other major debated issue in ischemic cardiomyopathy is the treatment of mitral regurgitation (MR). There is a complete agreement about the need to treat mitral regurgitation as an important factor of progressive remodelling, but the questions if it is better to repair or replace the valve and which grade of MR has to be considered the cut-off for surgical indication are still opened.
A milestones in this topic is the recent report of randomised trial NCT00807040 [1] in patients with severe mitral regurgitation. This study compares 251 patients randomly assigned to mitral-valve repair or replacement and followed for 2 years, assuming left ventricular end-systolic volume index (LVESVI) as the primary end point. All mitral replacements were performed with the preservation of the chordal apparatus and all mitral repairs were performed by means of downsized, restrictive anuloplasty (average ring size was 27.9 mm). As regards to the primary endpoint, the patients with recurrence in the repair group showed no reverse remodeling, as compared with those without recurrence (LVESVI of 64.1 ± 23.9 and 47.3 ± 23.0, respectively). Authors observed no significant between-group difference in left ventricular reverse remodeling or survival at 2 years. Mitral regurgitation recurred more frequently in the repair group, resulting in more heart failure related adverse events and cardiovascular admissions. As a negative outcome, as much as 58.8% of patients in this group had moderate or severe regurgitation during the follow-up period, as compared with 3.8% in the replacement group. Authors correctly judged this deficiency in the durability of mitral valve repair as “disconcerting”.
The high number of MR recurrences is a negative datum in absolute terms, more than compared to the group of mitral valve replacement. In fact, this last group could not relapse MR unless if due to a new problem (leaks, endocarditis, technical failure), not linked to the remodelling process. But in the repair group such frequent recurrences point to an ineffective surgical correction. Nevertheless, limitations in the effectiveness of restrictive anuloplasty had already been well assessed by several not randomised studies [1-4].
Authors conclude proposing a better selection of patients for repair by identifying baseline clinical or echocardiographic predictors of recurrence of mitral regurgitation.
As for the STICH trial, this study indirectly demonstrated some important evidences: LVESVI is at the same time the favouring factor and the result of an inverse remodeling; mitral repair is jeopardized by ventricular and valvular mechanisms different from annular restriction; several preoperative geometric measures could predict MR recurrence; results reflect a nonspecific patients’ selection that collects under the same “macroscopic” feature many valves and ventricles different in structural and functional characteristics.
A more comprehensive view
Is evident that the problem of decision-making process in ischemic cardiomyopathy is more complex than we considered until today and requires a wider view of ventricular structure and function. For decades we are used to judge the performance of the heart just in term of ejection fraction, disregarding other important factors. We categorised patients on this value, that is a basic ratio between two grossly estimated volumes, with high mathematical rounding and poor anatomical correlation. We also selected patients on the grade of mitral regurgitation, neglecting other structural data that identify several different types of mitral valves, with different potentialities / limitations to be corrected by a standard surgical procedure. Scientific knowledge often goes on for parallel roads and the discoveries achieved in some aspects are not always been rapidly integrated in a more complete vision. Nowadays we are required to have a wider vision of the heart, taking into account all its anatomical, structural, geometric and functional aspects, combined with a deep basic science knowledge.
In this wider vision, the role of myocardial fibers’ orientation and its direct functional expression, left ventricular torsion, recently gained attention and interest both in normal hearts and in pathologic conditions, including ischemic cardiomyopathy [1-7]. Until now, torsion was never found in any technique of ventricular reconstruction, while some papers dealing with recent or current techniques (Batista, SVR) actually reported a negative or neutral effect of surgical restoration on LV torsion itself [7,8].
We recently demonstrated [1,2] the unexpected potentiality to restore ventricular torsion after surgical treatment for ischemic cardiomyopathy. We devised a new technique of left ventricular reconstruction aimed at redirecting myocardial fibers to an almost normal setting (KISS procedure), obtaining the renewal of left ventricular torsion in a consecutive series of patients affected by chronic, severe ischemic cardiomyopathy [9,10]. Renewed torsion, as expression of restored fibers’ orientation and good global ventricular function and efficiency, could contribute to achieve systolic contraction and diastolic relaxation with a lower energy consumption [9,10], mimicking its role in normal hearts. This can help these ventricles that work at a critical level in Frank-Starling relationship and pressure-volume loop.
Given that myocardial fibers’ bundles branch out also in papillary muscles, we should rethink the strict connection between mitral valve and the left ventricle from this perspective. Structural dysfunction of myocardial bundles and even altered torsion itself can interfere with the normal functioning of the mitral valve.
As a consequence of this approach caring for global fibers’ realignment, we demonstrated:
- a long lasting physiologic ventricular reconstruction;
- no impairment in diastolic function;
- no new onset mitral regurgitation;
- a time-dependent inverse remodelling [9,10].
Fiber-based physiology
The function of every organ is closely correlated with its histology and anatomy. The structure of the heart, derived by its fascinating embryological evolution, is one of the most complex histological and anatomical architectures of a moving organ [11-20]. The basis of this structure are myocardial fibers and fibers’ bundles and their 3D disposition in interlaced, differently-oriented layers. We must actually keep it in mind in any clinical or instrumental visit to a patient. It is a concept based on the real functional basis of the heart. Thanks to cited 2D and 3D speckle tracking echocardiography, we can now look at the heart with this “new” interpretation, about 400 years after William Harvey, who stated in 1628 that “the motion of the heart consists of a tightening all over, both contraction along the fibers, and constriction everywhere” [9] (Figure 2).

Figure 2. Original latin text from “De Motu Cordis”, W. Harvey, 1628.
This new vision obviously applies to any kind of myocardial disease. Any single cause of cardiomyopathy interferes with the normal fibers’ architecture, leading to various degrees of fibers’ disarrangement, whether altering their orientation (dilated), or damaging their continuity (ischemic) or reducing their elasticity (inflammatory and toxic) [20-30].
Clinical perspective
Many studies highlight that clinical outcome is often limited by a partial correction of a complex disease. We should adapt our way of treating the heart to its high complexity, because its normalcy is a complex mix of many features. The Heart Team based, multidisciplinary treatment recommended in Heart Failure guidelines of American Heart Association [9] ultimately suggests to restore as much physiologic features as possible, driven by the entire set of normal parameters, guaranteeing at the same time the contemporaneity of treatments: otherwise, the benefit of any single correct therapy could be limited by the remaining features not yet corrected.
Fiber-based structure of the heart is the lowest common denominator among every myocardial disease, in an increasing-complexity scale going from sarcomere length to fibers’ bundles interaction. Left ventricular torsion is a key feature of normalcy and a strong predictor of positive outcome. The possibility to recover torsion by means of a surgical suture acting on residual myocardium is the most recent improvement in the comprehension of ischemic myocardial disease. The lack of consideration / use of this functional parameter could explain several failures in the surgical history of this cardiomyopathy [30-42].
A fiber-based physiology could also focus the functional connection between mitral valve and the left ventricle: adding regional contraction and rotational mechanics involving papillary muscles to the geometric features of an ischemic MR will identify hidden mechanisms which would otherwise unravel only after a partial correction. In this respect, the positive role of torsion has been recently confirmed also in a study about nonischemic, chronic, severe secondary MR [9,10]. This is a very important datum, towards an integrated view of a common left ventricular physiology linking different pathologic conditions.
A perfect knowledge of normalcy and a sharp modeling of disease will help us to reach a global correction of all altered parameters and obtain a better and long lasting correction of any cardiomyopathy.
References
- Sengupta PP, Korinek J, Belohlavek M, Narula J, Vannan MA, et al.(2006) Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol 48: 1988-2001
- Cooley DA, Collins HA, Morris GC, Jr, Chapman DW (1958) Ventricular aneurysm after myocardial infarction; surgical excision with use of temporary cardiopulmonary bypass. J Am Med Assoc 167: 557–560.
- Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, et al. (2009) Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 360: 1705-1717. [Crossref]
- Dor V, Kreitmann P, Jourdan J (1958) Interest of ‘physiological’ closure (circumferential plasty on contractile areas) of left ventricle after resection and endocardectomy for aneurysm or akinetic zone: comparison with classical technique of about 209 left ventricular resections. J Cardiovasc Surg 26: 73.
- Bolooki H, DeMarchena E, Mallon SM, Katariya K, Barron M, et al. (2003) Factors affecting late survival after surgical remodeling of left ventricular aneurysms. J Thorac Cardiovasc Surg 126: 374-383. [Crossref]
- Kono T, Sabbah HN, Stein PD, Brymer JF, Khaja F (1991) Left ventricular shape as a determinant of functional mitral regurgitation in patients with severe heart failure secondary to either coronary artery disease or idiopathic dilated cardiomyopathy. Am J Cardiol 68: 355-359. [Crossref]
- Di Mauro M, Iacò AL2, Bencivenga S3, Clemente D3, Marcon S3, et al. (2015) Left ventricular surgical remodelling: is it a matter of shape or volume?. Eur J Cardiothorac Surg 47: 473-479. [Crossref]
- Buckberg G, Athanasuleas C, Conte J (2012) Surgical ventricular restoration for the treatment of heart failure. Nat Rev Cardiol 9: 703-716. [Crossref]
- Adhyapak 2021 Copyright OAT. All rights reservof the left ventricle: insights for optimal surgical ventricular restoration. Heart Fail Rev 15: 73-83.
- Isomura T, Hoshino J, Fukada Y, Kitamura A, Katahira S, Kondo T et al. (2011) Volume reduction rate by surgical ventricular restoration determines late outcome in ischaemic cardiomyopathy. Eur J Heart Fail 13: 423-431.
- Suma H, Anyanwu AC (2012) Current status of surgical ventricular restoration for ischemic cardiomyopathy. Semin Thorac Cardiovasc Surg 24: 294-301. [Crossref]
- Buckberg GD, Athanasuleas CL (2009) The STICH trial: misguided conclusions. J Thorac Cardiovasc Surg 138: 1060-1064. [Crossref]
- Athanasuleas C, Buckberg G2, Conte J3 (2015) The STICH trial data: Keep it simple. J Thorac Cardiovasc Surg 149: 1682-1683. [Crossref]
- Michler RE, Rouleau JL, Al-Khalidi HR, Bonow RO, Pellikka PA, Pohost GM et al. (2013) Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg 146: 1139-1145.
- White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, et al. (1987) Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 76: 44-51. [Crossref]
- Raman J, Dixit A, Bolotin G, Jeevanandam V (2006) Failure modes of left ventricular reconstruction or the Dor procedure: a multi-institutional perspective. Eur J Cardiothorac Surg 30: 347-352. [Crossref]
- Buckberg GD, Athanasuleas CL, Wechsler AS, Beyersdorf F, Conte JV, et al. (2010) The STICH trial unravelled. Eur J Heart Fail 12: 1024-1027. [Crossref]
- Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, et al. (2016) Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med 374: 344-353. [Crossref]
- Magne J, Sénéchal M, Mathieu P, Dumesnil JG, Dagenais F, et al. (2008) Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol 51: 1692-1701. [Crossref]
- Ciarka A, Braun J, Delgado V, Versteegh M, Boersma E, et al. (2010) Predictors of mitral regurgitation recurrence in patients with heart failure undergoing mitral valve annuloplasty. Am J Cardiol 106: 395-401.
- Matsunaga A, Tahta SA, Duran CM (2004) Failure of reduction annuloplasty for functional ischemic mitral regurgitation. J Heart Valve Dis 13: 390-397. [Crossref]
- Zhu F, Otsuji Y, Yotsumoto G, Yuasa T, Ueno T, et al. (2005) Mechanism of persistent ischemic mitral regurgitation after annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation 112: I396-401.
- Shaw SM, Fox DJ, Williams SG (2008) The development of left ventricular torsion and its clinical relevance. Int J Cardiol 130: 319-325. [Crossref]
- Rüssel IK, Götte MJ, Bronzwaer JG, Knaapen P, Paulus WJ, et al. (2009) Left ventricular torsion: an expanding role in the analysis of myocardial dysfunction. JACC Cardiovasc Imaging 2: 648-655. [Crossref]
- Sengupta PP, Khandheria BK, Narula J (2008) Twist and untwist mechanics of the left ventricle. Heart Fail Clin 4: 315-324. [Crossref]
- Buckberg GD, Hoffman JI2, Coghlan HC3, Nanda NC3 (2015) Ventricular structure-function relations in health and disease: Part I. The normal heart. Eur J Cardiothorac Surg 47: 587-601. [Crossref]
- Buckberg GD, Hoffman JI2, Coghlan HC3, Nanda NC3 (2015) Ventricular structure-function relations in health and disease: part II. Clinical considerations. Eur J Cardiothorac Surg 47: 778-787. [Crossref]
- Buckberg G (2012) Outcomes of left ventricular reconstruction when established parameters are followed, and subsequent questions. Eur J Cardiothorac Surg 42: 393-397. [Crossref]
- Setser RM, Kasper JM, Lieber ML, Starling RC, McCarthy PM, et al. (2003) Persistent abnormal left ventricular systolic torsion in dilated cardiomyopathy after partial left ventriculectomy. J Thorac Cardiovasc Surg 126:48-55.
- Setser RM, Smedira NG, Lieber ML, Sabo ED, White RD (2007) Left ventricular torsional mechanics after left ventricular reconstruction surgery for ischemic cardiomyopathy. J Thorac Cardiovasc Surg 134:888-96.
- Cirillo M, Villa E, Troise G (2008) Improvement of left ventricular function after modified surgical ventricular restoration: good, better, best. Heart Surg Forum 11:E266-9.
- Cirillo M, Campana M, Brunelli F, Tomba MD, Mhagna Z, Messina A et al. (2010) “Let's twist again”: surgically induced renewal of left ventricular torsion in ischemic cardiomyopathy. J Cardiovasc Med (Hagerstown) 11:34-9.
- Cirillo M, Arpesella G (2008) Rewind the heart: a novel technique to reset heart fibers' orientation in surgery for ischemic cardiomyopathy. Med Hypotheses 70: 848-854. [Crossref]
- Cirillo M (2009) A new surgical ventricular restoration technique to reset residual myocardium's fiber orientation: the "KISS" procedure. Ann Surg Innov Res 3:6.
- Grosberg A, Gharib M, Kheradvar A (2009) Effect of fiber geometry on pulsatile pumping and energy expenditure. Bull Math Biol 71: 1580-1598. [Crossref]
- Esch BT, Warburton DE (2009) Left ventricular torsion and recoil: implications for exercise performance and cardiovascular disease. J Appl Physiol 106: 362-369. [Crossref]
- Cirillo M, Campana M, Brunelli F, Dalla Tomba M, Mhagna Z, et al. (2016) Time series analysis of physiologic left ventricular reconstruction in ischemic cardiomyopathy. J Thorac Cardiovasc Surg 152: 382–391.
- Buckberg GD (2016) Physiologic left ventricular reconstruction: Shape, function, and time recaptured. J Thorac Cardiovasc Surg 152: 392-393. [Crossref]
- Harvey W (1975) Classic pages in obstetrics and gynecology. Exercitatio anatomica de motu cordis et sanguinis in animalibus. Am J Obstet Gynecol 121: 1007. [Crossref]
- WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, Bozkurt B, Butler J, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: e240-327. [Crossref]
- Notomi Y, Isomura T, Kanai S, Maeda M, Hoshino J, et al. (2016) Pre-Operative Left Ventricular Torsion, QRS Width/CRT, and Post-Mitral Surgery Outcomes in Patients With Nonischemic, Chronic, Severe Secondary Mitral Regurgitation. J Am Coll Cardiol Basic Trans Science 1:193–202.
- Burkhoff D, Guccione J (2016) A New Twist on Mitral Regurgitation. J Am Coll Cardiol Basic Trans Science 1:203-206.


