Lichen Planus (LP) is a chronic mucocutaneous disease with several variants defined by the configuration of lesions, the morphological appearance, and the site of involvement. Although exact cause of LP is not known, it is thought that it is most likely an immunologically mediated reaction especially a cell-mediated immune response of unknown origin. Typical lesion is seen as a pruritic, papular eruption characterized by its violaceous color; polygonal shape; and, sometimes, fine scale. The size can vary from 1 mm to greater than 1 cm in diameter . It is most commonly found on the flexor surfaces of the upper extremities, on the genitalia, and on the mucous membranes. The clinical presentation of the disease has several forms: actinic, annular, atrophic, erosive, follicular, hypertrophic, linear, pigmented, vesicular/bullous and eruptive/exanthematous. “Exanthematous” or “eruptive” LP is a rare variant of the disease. Herein we report a 15 year-old boy, and a 32-year old man with a 1.5 and 3-month history respectively, of acute, episodic and eruptive mucocutaneous LP after a toluene exposure. To the best of our knowledge, our patients are the first cases of exanthematous mucocutaneous lichen planus that were caused by toluene exposure.
exanthematous, lichen planus, toluene
LP is a chronic inflammatory mucocutaneous disease of unknown etiology [1]. Even though the exact cause is still unknown, various precipitating factors including drugs and viral infections were speculated for the pathogenesis of the different forms of LP. Although these factors are known to play an important role in the pathogenesis, whether the targeted antigen is a virus or a drug is not known [1,2]. In the pathogenesis of the disease, it has been suggested that the availability of a given genetic predisposition, and the incorporation of a certain defined antigen may be considered to be the key factor. In fact, some patients with lichen planus have a positive family history. A significant relation with certain HLA phenotypes is considered to be possible, but it cannot be proved in most of the cases [3]. Another factor that has been argued about is autoimmunity, and the association of lichenoid dermatoses with autoimmune diseases, such as vitiligo, lupus erythematosus, alopeci areata, multiple sclerosis, ulcerous colitis, primary biliary cirrhosis, diabetes mellitus [4,5], and celiac disease [6].
A 15-year old boy was brought to our outpatient clinic because of his generalized and pruritic mucocutaneous eruption. The boy stated that he was inhaling liquid glue (®Bally) from a bag every 2-3 days and an acute eruption began after the 5th inhalation 1 month before. 6 hours after the last inhalation, firstly his lips became swollen, then he felt numb and extremely itchy, and finally vomitted. He also mentioned hallucinating. His parents brought him to the emergency service room at our hospital. The physical examination findings were hypotension, hypothermia, tachycardia, arrhythmia. In the dermatological examination, there was redness in both conjunctiva and an angioedematous swelling and bruising on his lips, palmar and plantar areas, and genitalia. In the full blood count, there were a slight leukopenia (3.2*103 mm3) and moderate eosinophilia (2.7*103 mm3). C reactive protein (CRP) level was 24 mg/L. The levels of IgE and the other immunoglobulins within the normal limits while anti-nuclear, anti-ds and anti-SS DNA antibodies were negative. No other pathology was detected. Within a five day period after the first intervention, the findings of the patient were completely resolved with slight hyperpigmentation and desquamation. However, the boy inhaled the same substance again three days before. Thereafter, an eruptive mucocutaneous rash began from his lips and palms insidiously, and spread to the whole body gradually. The patient was brought to our outpatient clinic. The physical examination of the patient was normal. The patient did not have atopic diathesis nor were there any clinical findings. In the dermatological examination, numerous, discrete, ill-defined, purple-colored, smooth and shiny-surfaced, 1mm to 3 mm in sized, polygonal papules on a diffuse livid macular backround were seen on the trunk, extremities and especially back of the fingers. Similar lesions, and a severe desquamation on the palmoplantar areas and genitalia were also found. The lips were edematous, and there were numerous punctate white papules and Wickham lines on the inner surface of the cheeks and lips (Figures 1a-1f). Hairs and nails were protected. Hematological and biochemical investigations, and serum total IgE level were similar to at the previous admission. Histopathology of a purple-colored papule revealed epidermal hyperplasia and acanthosis with wedge-shaped hypergranulosis, and a saw-toothed appearance of the undersurface of the epidermis in which there were necrotic eosinophilic keratinocytes and vacuolar alteration in the basal layer. In the upper dermis there was a dense, band-like lymphocytic infiltrate (Figure 2a). With the findings, the patient was diagnosed with LP. The patient was treated with one dose of intramuscular systemic steroid (Betametazon dipropiyonat plus betametazon sodyum fosfat), topical corticosteroid (flucticasone fluroate cream 0.05%) and an oral antihistamine (levocetirizine dihydrochloride). The exanthem was completely resolved in a three week follow up period. The patient was offered a challenge test with a patch test allergen which includes TDI (toluene diisocyanate), but his parents did not give consent.
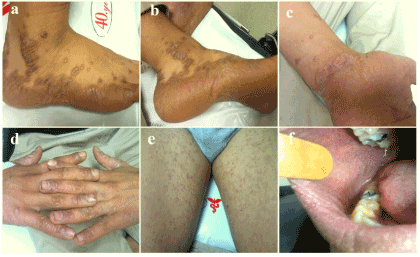
Figure 1. Skin and oral mucosa findings of the first patient (a,b,c,d,e,f).
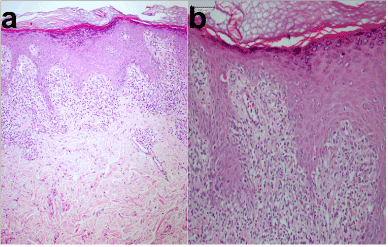
Figure 2. Histopathologies of the both patient. a) First patient (HEX100) and b) Second patient (HEX200)
A 32-year old man was admitted to our outpatient clinic because of his widespread and pruritic mucocutaneous eruption. He stated that his eruption began three months before from only his lips and fingertips. In the medical history of the patient, he first noticed the lesions two weeks after beginning his new job at a paint thinner factory and they gradually spread throughout the body. Additionally, he also felt moderate respiratory distress while working and the distress gradually increased. The dermatological examination showed deep and severe erythema and a thick desquamation on the palmoplantar areas, and flat-topped and dome-shaped polygonal, and shiny, purplish papules on the trunk, extremities and especially lateral sides of the feet and wrists. While small whitish papules were seen on the lips, both these papules as well as Wichkam lines were detected on the oral mucosa (Figures 3a-3f). Genitalia, nails and hairs were intact. The physical examination and routine laboratory tests of the patient were unremarkable excluding slight peripheral eosinophilia (2.9*103 mm3) and CRP (20 mg/L ). No atopic history or finding was detected. The levels of IgE and the other immunoglobulins within the normal limits. Anti-nuclear, anti-ds and anti-SS DNA antibodies were negative. The histopathological examination of a punch biopsy specimen showed, prominent epidermal hyperkeratosis and some acanthosis, hypergranulosis, and a saw-toothed appearance in which there were a few necrotic eosinophilic keratinocytes, and vacuolar changes along the basal layer. In the upper dermis, a dense, band-like lymphocytic inflammatory infiltration was seen (Figure 2b). The patient was diagnosed with LP, and he was also treated with one dose intramuscular systemic steroid (Betametazon dipropiyonat plus betametazon sodyum fosfat), topical corticosteroid (flucticasone fluroate cream 0.05%) and an oral antihistamine (desloratadine) . Additionally, the patient was given advice to leave and change his work, which he followed. The exanthem was completely resolved in a 1.5 month period. Three months after the recovery period, the patient was offered a challenge test with TDI, but he did not accept.
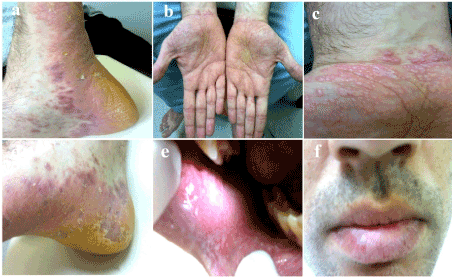
Figure 3. Skin, oral mucosa and lip findings of the second patient (a,b,c,d,e,f).
LP is typically seen as pruritic, faintly erythematous to violaceous, flat-topped, polygonal papules distributed mainly over the flexural areas of wrists, arms, and legs. The coalescence of individual papules into plaques, the presence of fine white lines on the surface of skin lesions, and the hyperpigmentation after resolution are its clinical characteristics [1]. Except for the skin, the disease can involve mucous membranes, hair follicles [7] and nails. Laryngeal, esophageal and conjunctival involvement rarely can occur. [8]. There are many clinical manifestations of LP such as annular, linear, hypertrophic, nodular, atrophic, bullous, erosive, actinic, follicular, mucosal and nail lichen planus. Cutaneous lesions can be localized or eruptive [9]. “Eruptive” or “exanthematous” LP is a uncommon variant of LP and it is rarely reported in the English-language literature, especially among adults [1]. In this variant, typically, the individual lesions are for the most part distinct. However, some of them can coalesce. The sites of predilection include the flexor surface of the wrists and the back of the feet. The other involved sites are neck, forearms, shins and anogenital region. If the trunk is involved, the disease becomes more acute and disseminates rapidly. This variant almost never involves the face and may rarely evolve into erythroderma [9]. The exact pathogenesis of LP is still unknown although as a causal role, many etiological factors have been accused of the pathogenesis such as drugs, gold salts, amalgam or composite resin, flavorings, denture components, viral and bacterial infections, emotional stress [1,8,9] and color film developing agents [9,10]. The molecular pathogenesis of LP is somewhat understood. It is thought that it can be a cell-mediated autoimmune reaction against basal layer keratinocytes [9]. The nature of autoantigens is unknown. However, it has also been discussed that the recurrent infections and the polymedication, are probably the cause for immune response expansion towards the cutaneous structures, imitating or exhibiting close morphology to bacterial, fungal or viral structural elements. Even though the relationship between the disease and psychological status, genetic or hormonal factors is unclear, Tchernev et al. stated that the patients may be predisposed to certain defined antigens and the antigens may also be due to permanent disturbances touching upon T- and/or B-cellular immunity or the macrophage system [3]. In the eary lesions, there is an abundance of antigen presenting cells, primarily Langerhans cells which can attract themselves through aberrant cytokine production or activate T cells which produce proinflammatory cytokines [9]. Primary T-cellular infiltrates in patients with lichen planus are initially compounded by CD4+, and later, by CD8+ lymphocytes [3]. In this cascade, INF-gamma induces class II MHC molecules, intercellular adhesion molecules (ICAM-1), and the cell dead receptor [Fas]. Class II MHC enhances T cell activity while ICAM-1 allows the attachment of lymphocyte-function-associated antigen [LFA]-1, a T cell surface protein. Thus, the keratinocytes are targets for T-cell mediated destruction via Fas-1 [activating ligand for FAS], perforins and granzyme B [3,9]. On the other hand, the concept of antigen mimicry means the activation of T- or B- cellular immunity with respect to certain cutaneous structures which are similar or analogous to exogenously introduced or secondarily and endogenously originated structures. Different bacterial, viral and parasitic infections, as well as the prescribed medicamentous therapies lead to disorders in the internal homeostasis. It is very interesting that drugs and bacterial or viral agents are able to provoke antigen mimicry, directly or indirectly [3]. Several diseases have been reported to be possibly associated with the mechanism of molecular mimicry, such as insulin dependent diabetes, multiple sclerosis, myasthenia gravis, pemphigus, Lyme diseases, syphilis, celiac disease, autoimmune uveitis [11,12]. Moreover, Bramanti, Sugerman and Chaiyarit, identified up-regulated heat-shock protein (HSP) expression by oral LP (OLP) lesional keratinocytes. Sugerman et al. speculated that keratinocyte HSP expression in OLP may be an epiphenomenon associated with pre-existing inflammation or up-regulated HSP expression by oral mucosal keratinocytes may be a common final pathway linking a variety of exogenous agents (systemic drugs, contact allergens, mechanical trauma, bacterial or viral infection) to be the pathogenesis. They also stated that in this context, HSP expressed by oral keratinocytes may be auto-antigenic in OLP [8]. In our patients, their rapidly evolving exanthems emerged after an exposure to glues included toluene. According to the patients histories, for the first patient, the time between the exposure and existence of the eruptions was 6 hours after the fifth exposure after regular inhalation every 2-3 days. In the second patient, the prodromal time was two weeks. These durations were suggest that emergence of the sensitizations lasted an average of two to three weeks. On the other hand, skin sensitization resulting in allergic contact dermatitis is by far the most frequent manifestation of immun toxicity in humans and a common occupational disease. This sensitization is a T cell-mediated delayed type hypersensitivity response to a low molecular weight reactive chemical called hapten. Skin sensitization develops in two distinct phases. The first is induction phase includes several events following the first contact with hapten and is completed when individual is sensitized and capable of giving a positive allergic reaction. The challenge phase begins upon elicitation by hapten and results in the clinical symptoms. Skin sensitizers are generally classified into T hepler type 1(Th1) or T hepler type 2 (Th2) depending on the induced cytokine profile [13]. On the other hand, exposure to volatile solvents like toluene, may be caused by an occupational exposure in industrial settings or a volatile substance abuse [14]. Toluene diisocyanate (TDI) is a very toxic volatile substance that is an aromatic hydrocarbone [15], and it is also a Th2 skin sensitizer [13] and is a weak skin irritant [16]. There are many report about the effect of chemicals on skin sensitization response [17,18]. TDI is considered one of many chemicals that is both a weak skin sensitizer and an irritant [19]. According to the U.S. Occupational Health and Safety Administration, the safe limit allowable for solvents is 200 parts per million (ppm) for toluene. But, the concentration of toluene inhaled from a bag which contains Bally might reach 10,000 ppm [14]. The smell of toluene provides sufficient warning for dangerous concentrations of toluene, exposure to toluene however, if lasts for 15 minutes at a concentration of 8 ppm, which is the sensory threshold value, it leads to olfactory insufficiency. Severe toluene exposure may cause fluid deposition in the lungs and respiratory arrest. Chemical pneumonitis may develop as a result of pulmonary aspiration in the course of liquid toluene ingestion, or vomiting after ingestion. Repeated and long term cutaneous exposure of toluene may cause erythema and urticaria. Toluene exposure-related ocular inflammation is usually mild. Burning, conjunctivitis, and keratitis may develop when toluene comes into contact with the eye by accidental splashing [15]. The common feature in the histories of both our cases was that they inhaled substances that incude toluene. In the first patient, after an approximately 3 week sensitizasyon time, the clinical symptoms began with numbness, itchiness and, swollen and bruised lips, palmar, plantar, and genital area in addition to vomitting, hallucinating, hypotention, hypothermia, tachycardia and arrhythmia. Furthermore, there were slight leukopenia and moderate eoinophilia. CRP level was high. These findings suggest that the toluen exposue affected the brain, cardiovascular system, respiratory tract and bone marrow, and the repeated exposures caused an eruptive LP. Except for the conjunctival redness and angioedematous swellings, the skin lesions of our first patient did not resemble the findings of toluen toxicity. On the other hand, in our second patient the clinical symptoms began following a 2 week sensitization time. He had moderate respitatory distress, slight eosinophilia, and high CRP level. These clinical findings matched the signs associated with toluen toxicity. Additionally, the findings of the dermatological examination, resembled those of our first patient. Therefore, we thought that the skin findings were caused by exposure to toluene. Moreover, after the exposure to toluen was ceased, all the findings of both patient gradually resolved completely. Patient 1 recovered within 3 weeks of starting therapy while patient 2 recovered witin a one and half month period. Compared to general T-cell sensitization time, which usually last 4 weeks, our patient’s prodromal times were much shorter. Because of the toluen is not a strong sensitizer, we think that the reason for its shortness stemmed from the high toxicity level toluene. Unfortunatelly, we could not test to toluen toxicity of either patient because neither gave consent. Diisocyanates including TDI, although associated with dermal sensitization are not among the commonly implicated substances for which standardized concentrations have been developed. However, there have been only a handful of reports of ACD due to diisocyanates [20]. Kanerva et al. have stated that in a series of 360 patient, TDI was found the elicit an allergic response in 0.8% while inducing an irritant reaction in 1.9% of the cases [21]. On the other hand, diisocyanates are among the substances for which no generally accepted, standardized patch test method has been adopted. Various concentrations have been recommended and used. For TDI, concentrations have ranged from 0.1 to 2 percent [22]. As an example of the difficulty of interpreting patch testing results, one case has been reported to have a negative result with TDI at 0.1 percent while at 0.5 percent concentration a positive result was seen [16]. Therefore, it is not always possible to differentiate between irritant and allergic contact dermatitis through patch testing [16,20,21]. Furthermore, Littorin et al.. have reported a patient who had been working with isocyanate glue (it was diphenylmethane which is another diisocyanate) for about a week. Their patient had a very high total IgE, specific IgG (G1 and G4), eosinophilia and moderate neutropenia, fever, myalgia, shivering and serious pulmonary symptoms but no skin reaction. After the patient had started to use a new glue (“super-epoxy”) he was developed some skin rash and papular eruptions on his hands, and facial edema. He had some otoimmune conditions such as crescentic glomerulonephritis, optic neuritis, anti-C1q, anti-SS-A and anti-myeloperoxidase antibodies. The authors indicated these findings support to their patients autoimmune disposition. However, although detailed examinations they also stated that the mechanism behind the isocyanate-related disease was still obscure [23]. In our patients we did not find additional disease or laboratory findings which can support to an autoimmune disposition. In addition, during the one-year follow-up period their diseases did not recur and they have not exhibited any autoimmune disease so far, therefore we thought that their reactions had not an autoimmune basis. Despite the quite different clinical variants the histopathology of LP is relatively uniform. The key feature is a band-like infiltrare of T cells at the dermo-epidermal junction. The epidermis may be acanthotic with compact hyperkeratosis and focal wedge-shaped hyperplasia of the granular layer, which produce the Wickham striae. There is an achantosis in most lesions, however this can vary in hypertrophic and atrophic regions. In the basal layer vacuolar changes are seen along with colloid bodies, reflecting damaged often dyskeratotic keratinocytes. In older lesions, there is incontinence of pigment with melanin in dermal macrophages [9]. The histopathological findings of our patients had similar features to each other and complied with LP. In the differential diagnosis of disseminated and discrete papules and macules of LP, papular umbilicated granuloma annulare, atypical pityriasis rosea, verruca plana, pityriasis lichenoides and lichen scrofulosorum, and especially Lichenoid Drug Eruptions (LDE) should be considered [1]. Except for the last, LP can easily be distinguished from the others with typical histological features. LDE is a rare type (<1%) of drug eruption, and clinical features are similar to lichen planus [24]. Differentiating drug-induced lichen planus from the idiopathic form is difficult. In idiopathic lichen planus, flat-topped, shiny, violaceous papules with Wickham's striae and a predilection for the wrists, flexures, genitalia and mucous membranes are seen [25]. However, some features have been described to be more characteristic of LDE; usually symmetrical lesions on the trunk and extremities, atypical morphology, absence of Wickham's striae, rare mucosal involvement, healing with residual hyperpigmentation, focal parakeratosis, hypogranulosis, and a superficial and deep perivascular infiltrate, higher number of grouped necrotic keratinocytes, and infiltration of plasma cells, eosinophils [24] and neutrophils. Additionally, LDE may appear psoriasiform or eczematous, and affects older patients and photoexposed areas [25]. Both the clinical and histopathological features of our patients more complied with a LP than a LDE.
In conclusion, as mentioned earlier, some of the diseases can be associated with lichenoid dermatoses [4,5]. Based on this information, we suggest that an antigen mimicry may be responsible for the clinical picture of this rare type of LP. In this picture, a crossed-mediated reaction of T-cell type might have been occured between host keratinocytes and toluene-affected cells, and the toluene might have been etiologically guilty as it acted like a hapten, or an epiphenomenon that induced some HsPs from the affected cells. Additionally, possible genetic basis of the patients can provide their dispositions, and high respiratory toxicity of toluene might have been caused the faster and more severe skin reactions with cross sensitization similar to generalization an allergic contact dermatitis. Our case is being reported to draw attention to this rare entity and a probable association between toluene and eruptive LP. We also tried to interpret a possible etiopathological mechanism of the disease according to the concepts of molecular mimicry and HsPs. Unfortunately, we do not have enough and satisfying knowledge to explain the skin and other tissue reactions against toluene, at least now. More detailed information, and prospective/controlled future studies concerning immunopathogenesis of toluene-induced tissue responses are needed for more precise conclusions.
- Liu KC, Lee JY, Hsu MM, Hsu CK (2013) The evolution of clinicopathologic features in eruptive lichen planus: a case report and review of literature. Dermatol Online J 19: 8.
- Fleming J, Diaz-Cano S, Higgins E (2011) Eruptive lichen planus triggered by acupuncture. Arch Dermatol 147: 361-362.
- Tchernev G, Nenoff P (2009) Antigen mimicry followed by epitope spreading: a pathogenetic trigger for the clinical morphology of lichen planus and its transition to Graham Lassueur Piccardi Little Syndrome and keratosis lichenoides chronica - Medical hypotheses or reality? An Bras Dermatol 84: 682-688.
- Schuh T, Röcken M, Schmoeckel C, Degitz K (2002) Lichen ruber planus after hepatitis B vaccination. Hautarzt 53: 650-651.
- Rabinovich OF, Khanukova LM, Khamidulina KF (1999) The characteristics of the immune status of patients with lichen ruber planus. Stomatologiia (Mosk) 78: 20-23.
- De D, Kanwar AJ (2008) Eruptive lichen planus in a child with celiac disease. Indian J Dermatol Venereol Leprol 74: 164-165.
- Erdem T, Güleç AI, Aktaş A, Yildirim A, Kiziltunç A (2004) Increased serum level of p-selectin in patients with lichen planus. Yonsei Med J 45: 215-218.
- Sugerman PB, Savage NW, Walsh LJ, Zhao ZZ, Zhou XJ, et al. (2002) The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med 13: 350-365.
- Braun-Falco O, Plewing G, Wolf HH, Burgdorf WHC (2000) Erythema-papulo-squamous diseases, in: Dermatology. (2ndedtn). Springer-Verlag, Berlin
- Lidén C (1986) Lichen planus in relation to occupational and non-occupational exposure to chemicals. Br J Dermatol 115: 23-31.
- Oldstone MB1 (1998) Molecular mimicry and immune-mediated diseases. FASEB J 12: 1255-1265.
- Aberer E, Brunner C, Suchanek G, Klade H, Barbour A, et al. (1989) Molecular mimicry and Lyme borreliosis: a shared antigenic determinant between Borrelia burgdorferi and human tissue. Ann Neurol 26: 732-737.
- Morimoto T, Higaki T, Ota M, Inawaka K, Kawamura S, et al. (2014) Effect of simultaneous exposure to mixture of two skin sensitizers on skin sensitization response in guinea pigs and mice. J Toxicol Sci 39: 163-171./li>
- Büker HS, Demir E, Yüncü Z, Gülen F, Midyat L, et al. (2011) Effects of volatile substance abuse on the respiratory system in adolescents. Multidiscip Respir Med 6: 161-168.
- Karabulut I, Pinar T, Karabulut H, Melike D, Karadeniz G, et al. (2012) Skin prick test results and prevalence of allergic symptoms in workers exposed to toluene. Turk J Med Sci 42: 63.
- Ott MG, Diller WF, Jolly AT (2003) Respiratory effects of toluene diisocyanate in the workplace: a discussion of exposure-response relationships. Crit Rev Toxicol 33: 1-59.
- Wille JJ, Kydonieus A, Kalish RS (1998) Inhibition of irritation and contact hypersensitivity by phenoxyacetic acid methyl ester in mice. Skin Pharmacol Appl Skin Physiol 11: 279-288.
- Matsuda T, Maruyama T, Iizuka H, Kondo A, Tamai T, et al. (2010) Phthalate esters reveal skin-sensitizing activity of phenethyl isothiocyanate in mice. Food Chem Toxicol 48: 1704-1708.
- Nethercott JR, Ho2021 Copyright OAT. All rights reserv contact dermatitis. Clin Rev Allergy 7: 399-415.
- Lubach D (1978) Diseases caused by diisocyanates. 1. Irritation of the respiratory system and skin. Derm Beruf Umwelt 26: 184-187.
- Kanerva L, Jolanki R, Alanko K, Estlander T (1999) Patch-test reactions to plastic and glue allergens. Acta Derm Venereol 79: 296-300.
- de Groot AC, Frosch FJ (1992) Patch test concentrations and vehicles for testing contact allergens. In: Textbook of contact dermatitis (2ndedtn), Springer-Verlag; Berlin, Heidelberg. 795-805.
- Littorin M, Truedsson L, Welinder H, Skarping G, Mårtensson U, et al. (1994) Acute respiratory disorder, rhinoconjunctivitis and fever associated with the pyrolysis of polyurethane derived from diphenylmethane diisocyanate. Scand J Work Environ Health. 20: 216-222.
- Cho HK, Lee SY1 (2014) Prominent alopetic lesions of lichenoid drug eruption treated with topical tacrolimus. Ann Dermatol 26: 141-143.
- Kolm I, Eggmann N, Kamarashev J, Kerl K, French LE, et al. (2013) Lichenoid Drug Eruption following Intravenous Application of Orally Formulated Diamorphine, a Semisynthetic Heroin. Case Rep Dermatol 5: 176-180.



