Abstract
Rostral-caudal standing resonance waves over the human cerebrum have been hypothesized and demonstrated. Here we demonstrate the emergence of resonant current density profiles when the measures from the right and left lingual gyrus and right and left anterior cingulate were spectral analyzed for QEEG records from 237 normal volunteers. The first three peak frequencies of 7-8 Hz, 13-14 Hz and 19-20 Hz which are the same as the first three harmonics of the Schumann Resonances were evident for both cerebral regions. At higher harmonics the discrepancies between the anterior cingulates showed Δfs of 3 Hz rather than 6 Hz. The estimated right-left current discrepancies of the trough-to-peak spectral measures for the peaks were in the order of 10-8 A·mm-1 for the lingual regions and would be consistent with intrinsic magnetic field strengths in the pT range. These results indicate translational brain rhythmicity may emerge between hemispheres as “interference” or “beat frequencies” that are remarkably similar in harmonics and magnetic field intensity to the Schumann fields that are generated between the earth-ionospheric cavity.
Key words
QEEG, interhemispheric resonances, lingual gyrus, anterior cingulate, s_LORETA, Schumann resonances
Introduction
Contemporary approaches to the functional correlates, which include thoughts, images, and aberrant experiences, of dynamic activity within the cerebral volume have focused upon networks [1,2]. Implicitly these connections are relatively fixed within brain space and vary with respect to the proportion of participation from various components of the network as a function of exteroceptive and introspective conditions. The classic example is the Default Mode Network [3,4] with which subjective experiences and their strong personal meaningfulness is associated. Its vector is primarily in a rostral-caudal direction. Recently we have shown that recondite spectral power densities occur within normal quantitative electroencephalographic records that reflect a far greater translational capacity of the human cerebrum [5,6].
There is another domain of translational brain rhythmicity that is related to distance between structurally separated and sparsely connected regions within the cerebrum. These phenomena are emergent properties that occur as a consequence of resonance. Natural resonance of systems is typically calculated by an intrinsic velocity divided by the circumference or boundary of the structure. Many years ago the perspicacious Nunez [7] had shown that an intrinsic resonance or standing wave from a mean bulk velocity (~4.5 m·s-1) of rostral-caudal integrated fields over the cerebral cortices compared to the cerebrum’s circumference was between 7 and 8 Hz. This fundamental frequency may serve as the substrate for the current recreations of large-scale cerebral fields that occur every 20 to 25 ms [8,9] or between 40 and 50 Hz.
One major consequence of rostral-caudal movement of integrated, neurogenic fields is that prefrontal and organizational states would affect the cognitive configuration of the more perceptual functions of the caudal hemispheres. Stated in the vernacular, expectancy strongly influences what is perceived. These integrated fields move along a continuous surface boundary. The most conspicuous example of spatial disparity and discontinuity occurs in the orthogonal plane (left-to-right) and is manifested by the separation of the two cerebral hemispheres. Despite their apparent superficial similarity their geometries of gyral-sulcal patterns are quite distinct [10]. There are also significant differences in mass, in the order of a few tens of grams as well as ratios of white matter to grey matter in specific regions [11,12].
These heterogeneities of mass and constituency create the conditions to facilitate small discrepancies in complex electrocortical configurations that would produce emergent features that could be considered interhemispheric mosaics, interference patterns, “beat frequencies” or “derivative” fields that may serve as the substrate for complex neurocognitive processes and disease states. Here we present evidence, employing sLORETA (Low Resolution Electromagnetic Tomography) that specific spectral densities at fixed foci emerge from hemispheric discrepancies that could accommodate the persistent correlates of consciousness, specific disease conditions and the potential interaction with natural electromagnetic fields whose intensities and frequency profiles strongly converge with human quantitative electroencephalographic (QEEG) activity.
The most conspicuous environmental congruence is the Schumann Resonance [13] that is generated between the ionosphere and the earth’s surface by lighting activity that occurs at a frequency of about 44 ± 5 Hz [14]. The fundamental frequencies of these electromagnetic resonances are equal to 8 Hz, 14 Hz, 20 Hz, 26 Hz, etc with approximate increments (Δf) of 6 Hz. The amplitudes and small split frequency shifts can occur in response to a variety of geophysical variables [13]. The electrical component is ~1 mV per m and the magnetic field is ~1 pT (10-12 T). These values are the same orders of magnitude generated by cerebral cortical activity [5,6].
Experimental procedure
Over the last five years we have collected an extensive database of QEEG data under standardized conditions for 237 healthy individuals. The data were collected through the Mitsar-201 system employing a 19 channel EEG cap as described previously [5,6]. The full database (NED) has been published elsewhere [5] and can be accessed from the following web link: https://figshare.com/articles/Raw_19_channel_EEG_Data/1601695. For the present study two areas of interest were selected: the lingual gyrus and the anterior cingulate region. They were selected because of their central role within intrahemispheric networks involved with dreaming and experiences coupled to the sense of self and the internal states associated with self-reflection.
For each of the two analyses 16 s epochs of eyes closed baselines (the majority measured while subjects sat within a dimly lit acoustic chamber) were extracted. The raw 19 channel data were imported into sLORETA software [14] where time series sLORETA scores were computed. First, the EEG-to-sLORETA function computed current source densities for each of the 4,000 sample points that comprised the 16-second epochs for each subject (sample rate=250). Next, region-of-interest (ROI) seeds were created for the approximate locations of the bilateral lingual gyri (MNI coordinates: X ± 14, Y=-85, Z=-5) and anterior cingulate (MNI coordinates: X ± 3, Y=44, Z=1) within a radius of 10 cm for the lingual gyrus and 5 cm of the anterior cingulate. We selected these two regions because of their median distance within the frontal and caudal portions of the longitudinal axis.
Figure 1A illustrates the ROI seeds (depicted as yellow pixels) superimposed on sLORETA images. The sLOR-to-ROI function was then used to extract the ROI current source densities (μA·mm-2) for each discrete 4-millisecond increment from the bilateral lingual gyri and an anterior cingulate regions. The result of this transformation can be seen in Figure 1B which shows sample simultaneous ROI time-series for the bilateral anterior cingulate and lingual gyri for a single subject.
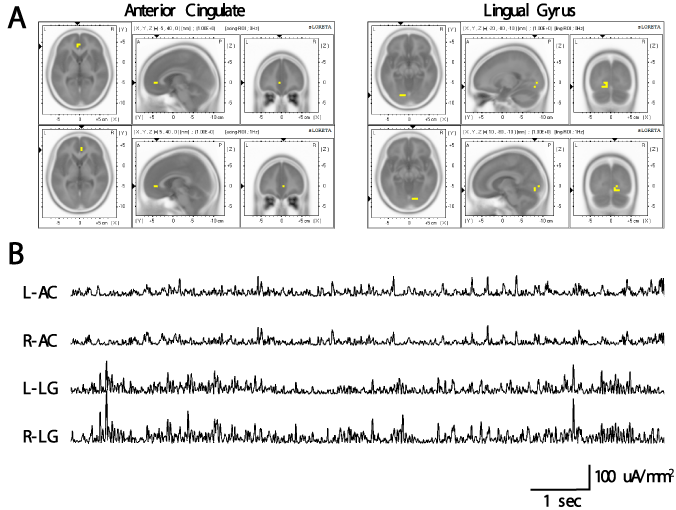
Figure 1. (A) Yellow pixels indicate regions of interest from the perspective of horizontal, saggital, and coronal for the left (top) and right (bottom) anterior cingulate (left) and lingual gyrus (right) extracted for the analysis. (B) Examples of sLORETA time series for the bilateral anterior cingulate and lingual gyrus for one subject. These time-series were later entered into spectral analysis. L-AC: left anterior cingulate; R-AC: right anterior cingulate; L-LG: left lingual gyrus; R-LG=right lingual gyrus.
These time series were imported into MATLAB where spectral analyses for each case were completed on the 4-channel data [left and right anterior cingulate (2 channels), left and right lingual gyrus (2 channels)] using the spectopo.m function within freely available EEGLab software [15] employing 2048 FFT points (0.061 Hz) to maximize spectral resolution within the 0-50 Hz frequency band. Grand averages of the spectral scores for all 4 channels were obtained by computing the mean across the 237 subjects for each discrete frequency. A moving average with a period equal to 15 (.915 Hz) was then applied to these averages. Net inter-hemispheric differences for each ROI were computed by subtracting the left from the right.
Results
As expected there was slightly greater current source density (CSD) over the right hemisphere in general. However when the current spectral densities (CSD2·Hz-1) from the left lingual gyrus region were subtracted from the spectral densities from the right lingual gyrus, a resonance-like effect was evident. There were significant peaks at approximately 8 Hz, 13 Hz, 18 Hz, 22 Hz, and 26 Hz. The trough-to-peak differences are equivalent to about 9.5·10-8 A·mm-2 after adjustment for log and multiplier functions. The results of the analyses were similar for the anterior cingulate, however the inter-harmonic spacing was separated by 3 Hz as opposed to 6 Hz observed for lingual gyrus. The other conspicuous feature involved the amplitudes of the resonances. The peak-to-peak amplitudes for the appropriately adjusted discrepancies between the right and left lingual regions were ~5 times larger than these values for the discrepancies between the right and left anterior cingulate regions. The results of this analysis have been depicted in Figure 2 that shows the inter-hemispheric resonances (adjusted for direct comparison) for the anterior cingulate and lingual gyri for direct comparison. The vertical axis shows the spectral densities for the current source densities [(μA·mm-2)2·Hz-1] inferred by sLORETA.
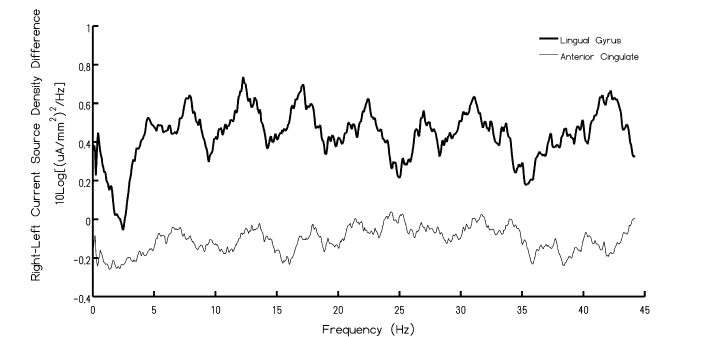
Figure 2. Spectral power of current density discrepancies between the right and left hemisphere for the lingual and anterior cingulate regions as a function of frequency. Note the peaks within the Schumann Resonance.
Discussion
The rostral-caudal resonance of 7 to 8 Hz for the human cerebrum as described by Nunez and demonstrated indirectly and directly by the 20-25 ms “refresh” rate for rostral-caudal transcererbal electromagnetic fields by Llinas et al [8] might be argued to reflect fixed physical parameters. These would include the more or less restricted volume of the human cerebrum as well as the prominence of rostral-caudal tract systems that integrate the organizational components of the brain with the more receptive and perceptional components of the brain activity.
Although there are tracts such as the corpus callosum, composed of about 250 million fibers, and the anterior commissure, which is dominated by inputs and outputs from the ventral temporal cerebral cortices (as well as mesiobasal temporal lobe subcortical structures) the spatial continuity that defines the rostral-caudal propagation is not present. Given there are approximately 23 to 25 billion cerebral cortical neurons [16], the numbers of axons that interconnect the two hemispheres through the corpus callosum would be in the order of 1%. This would suggest, considering the cross sectional area of the corpus callosum compared the areas of the medial surfaces of the hemispheres, that a marked spatial discontinuity as reflected in the longitudinal fissure would be potentially impeding.
Considering the remarkable microdiscrepancies of structural organization between the right and left hemispheres differences in QEEG amplitudes and intrinsic subtle frequency patterns would be expected. Our results, which involved the averages of more than 200 normal QEEG records collected over several years with standardized procedures indicate that when the right hemispheric power densities are subtracted from the left hemisphere power densities (sampling 250 Hz) as inferred by sLORETA localization employing MNI coordinates, emergent periodicities became visible and conspicuous. The first three harmonics at ~8 Hz, ~14 Hz, and ~20 Hz, were similar for both the lingual region and the anterior cingulate region. These are also the first three harmonics of the Schumann Resonance generated by the earth-ionospheric wave guide.
The trough-to-peak values for the current densities varied by a factor of about 5 between the two areas. Whether or not distance was the determining factor of the organization of the rostral-caudal interhemispheric patterns were more important must still be established. The estimated distance between the two ROI for the anterior cingulates was 6 mm while the distance between the two ROI for the lingual regions was 28 mm. This is approximately a factor of 5. Regardless of the etiology what is clear is that above the third harmonic the anterior cingulate discrepancies doubled the resonance peaks such that the Δf shifted from 6 Hz to ~3 Hz.
One frequent test to infer if measurements are valid is to discern if quantitative estimates converge. For the lingual regions, the peak-to-trough difference was equivalent to ~10-8 A over a cm which would be ~10-6 A·m-1. According to Nickolaenko and Hayakawa [10] the magnetic field measured in Tesla can be discerned by the relation 1 μA·m-1=0.4πpT. Hence the equivalent magnetic field strength would be about 2 pT. In addition if the classic equation for discerning a magnetic field were applied within a cylinder of space [that is B=μI (2πr)-1 where μ is magnetic permeability (4π·10-7 N·A-2), I is current, r is radius, and B is the magnetic field strength], the estimated ~9.5·10-8 A as a median value for the trough-to-peak value for the lingual region with a 10 mm radius would result in ~2·10-12 T or 2 pT. If the typical potential difference of the EEG band associated with cognition (~2·10-6 V) is divided by the resistivity of extracellular fluid (~2 Ω·m) and multiplied by the first order length of the cerebrum (~11 cm) based on its volume, the current is 10-7 A. When multiplied by magnetic permeability μ and divided by the median length of the cerebrum the emergent magnetic field dimension displays an intensity of 2 pT.
Although we understand the potential for coincidence, if the inference that the right-to-left hemisphere discrepancy in current density for the lingual regions for the Schumann peaks is indeed around 2 pT, then the potential for resonance interaction with the Schumann parameters might be considered a possibility. Our research [5,6] as well as the contributions of others [17,18] indicates that human beings on the surface of the planet are immersed in these fields. Direct measurement and coherence analyses indicate there is intermittent concordance about every 30 s for about one or two microstate durations (about 0.5 s) between the human QEEG and the Schuman conditions in real time measured here locally or in Italy [6].
These results may open a novel research area of translational brain rhythmicity that includes both intracererbal and intercererbral components associated with external sources that share human brain parameters [19]. Given the hemispheric asymmetry of distribution of classic neurotransmitters [20] there is the additional advantage of integrating chemical profiles with these electromagnetic properties. There have been historical and robust philosophical debates concerning the implications of a dominant rostral-caudal reiteration of cortical waves associated with consciousness, including aberrant, diseased, and normal variants. The vector strongly indicates that caudal cerebral perceptions are, by structural determination, organized by frontal activity and its functions such as expectancy. Our results indicate that the right hemispheric contributions to the left hemispheric functions associated with cognition, images, and insight might be influenced by global environmental variables such as the second-to-second variations of the information within the Schumann Resonances.
Acknowledgements
This work is dedicated to Dr. H. L. Koenig and Dr. Neil Cherry whose prescient concepts and research have been unappreciated.
References
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, et al. (2006) Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry 60: 1231-1240. [Crossref]
- Downar J, Crawley A2021 Copyright OAT. All rights reservimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3: 277-283. [Crossref]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009) Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19: 72-78. [Crossref]
- Collins MW, Persinger MA (2014) Enhanced power within the default mode network in normal subjects with elevated scores on an egocentric scale. Open Neuroimag J 8: 5-10. [Crossref]
- Saroka KS, Vares DE, Persinger MA (2016) Similar Spectral Power Densities Within the Schumann Resonance and a Large Population of Quantitative Electroencephalographic Profiles: Supportive Evidence for Koenig and Pobachenko. PLoS One 11: e0146595. [Crossref]
- Persinger MA, Saroka KS (2015) Human quantitative electroencephalographic and Schumann resonance exhibit real time coherence of spectral power densities: implications for interactive information processing. Journal of Signal and Information Processing 6: 153-164.
- Nunez PL (1995) Towards a physics of the neocortex. In. Nunez, P. L. (ed). Neocortical dynamics and EEG rhythms. Oxford University: N.Y., USA, pp. 68-130.
- Llinás R, Ribary U (1993) Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A 90: 2078-2081. [Crossref]
- Persinger MA (1999) Is there more than one source for the temporal binding factor for human consciousness? Percept Mot Skills 89: 1259-1262. [Crossref]
- Van Essen DC, Drury HA (1997) Structural and functional analyses of human cerebral cortex using a surface-based atlas. J Neurosci 17: 7079-7102. [Crossref]
- Blinkov SM, Glezer II (1968) The human brain in figures and tables: a quantitative handbook. Basic Books: N.Y.
- Büchel C, Raedler T, Sommer M, Sach M, Weiller C, et al. (2004) White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex 14: 945-951. [Crossref]
- Nickoaenko A, Hayakawa M (2014) Schuman Resonances for Tyros. Springer: Tokyo, Japan.
- Pascual-Marqui RD (2002) Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 24 Suppl D: 5-12. [Crossref]
- Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9-21. [Crossref]
- Crosby EC, Humphrey R, Lauer EW (1962) Correlative Anatomy of the Nervous System. The Macmillan Company, New York, USA.
- Koenig HL, Krueger AP, Lang S, Sonnig W (1981) Biologic effects of environmental electromagnetism. Speringer-Verlag: Berlin, Germany.
- Pobachenko SV, Kolesnik AG, Borodin AS, Kalyuzhin VV (2006) The Contingency of Parameters of Human Encephalograms and Schumann Resonance Electromagnetic Field Revealed in Monitoring Studies. Biophysics 51: 480-483.
- Persinger MA (1974) ELF and VLF electromagnetic field effects. Plenum Press: N.Y, USA.
- Kurup RK, Kurup PA (2003). Hypothalamic digoxin, hemispheric chemical dominance, and spirituality. Int J Neurosci 113: 383-393.


