Abstract
Penetrating cardiac injuries are commonly instantaneously lethal. However, numerous delayed manifestations of cardiac injury, including traumatic ventricular septal defect, ventricular aneurysms, right pulmonary artery to left atrial fistula, valvular regurgitation, and aorto-right ventricular fistula, are all described in the literature. Here, we present a case of aorto-right ventricular fistula following a stab wound to the chest. Epidemiology, diagnosis, imaging, and management of penetrating cardiac injury and intracardiac fistulas are discussed.
Key words
cardiac injury, intracardiac fistula, stab wounds, high output heart failure, aorto-right ventricular fistula
Introduction
Penetrating cardiac injury remains an area of great morbidity and mortality. Only 6% of victims of penetrating cardiac injury are expected to survive hospital transport [1,2]. Of those who survive, the complication rate is not negligible. In a retrospective review of 56 survivors of penetrating cardiac injuries followed for at least 6 months, 16 (29%) had delayed complications [3]. One rare and potentially lethal complication is a delayed aorto-right ventricular (RV) fistula. Here, we present the case of a 26-year-old male after a stab wound to the chest. He initially underwent emergent sternotomy and repair of a pulmonary artery laceration, and later developed a symptomatic aorto-RV fistula.
Case report
A 27-year-old man presented with a stab wound to the anterior cardiac box. He was tachycardic, hypotensive, and intubated for a Glasgow Coma Score (GCS) of 3T. Focused assessment with sonography in trauma (FAST) scan showed pericardial effusion and clot. He was taken to the operating room for a median sternotomy. A 3cm laceration to the anterior pulmonary artery was primarily repaired. The incision was extended to a left thoracotomy to ligate a bleeding intercostal artery.
Post-operatively, transthoracic echocardiography (TTE) performed for otherwise unexplained persistent bacteremia and fungemia demonstrated a fistula between the aortic root and the right ventricular outflow tract. There were no demonstrable vegetations and ejection fraction was estimated at 48% (Figure 1). Subsequent cardiac CT demonstrated four chamber enlargement and delineated the anatomy of a shunt between the right coronary cusp of the aorta to the pulmonary artery outflow tract, measuring 8mm x 6mm. Magnetic resonance imaging demonstrated a shunt fraction (QP/QS ratio) of 1.89, and a cardiac output of 11.26 L/min, indicating that the shunt was significant and causing high output heart failure.
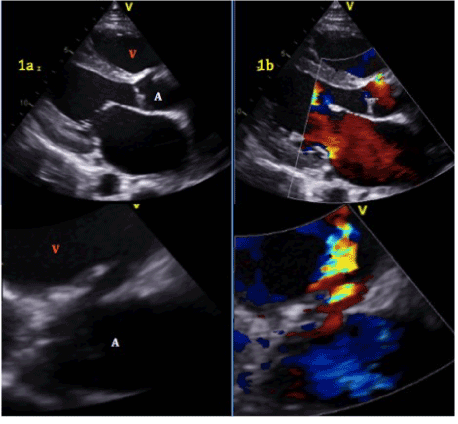
Figure 1.a.Aorto-right ventricular fistula. Aortic root (A), right ventricle (V).b. Left to right shunt with flow from aortic root to right ventricle.
The patient’s initial postoperative course was complicated by pneumonia and respiratory failure requiring tracheostomy. He completed a four-week course of intravenous vancomycin for methicillin-resistant Staphylococcus epidermidis bacteremia and a two-week course of fluconazole for Candida fungemia. Interval repair was planned to follow hematological bacterial clearance, and at a time when pericardial adhesions would not be prohibitive following initial surgery (approximately 6-8 weeks later). Although the patient required one interval inpatient stay for management of his heart failure, he responded well to diuresis and medical optimization prior to operation.
Two months after initial injury, the patient underwent definitive repair of his aorto-right ventricular fistula, approached by median sternotomy, extensive adhesiolysis, and central cannulation for bypass. Aortotomy revealed a pseudoaneurysm of the proximal aorta close to the original pulmonary artery injury. A 4x2mm aorto-right ventricular fistula from the right sinus of Valsalva just below the right coronary artery was identified (Figure 2). The defect was closed using bovine pericardium patch overlay (Figure 3) and no fistula was seen on post-operative echocardiography coming off bypass. The patient had an uncomplicated postoperative course and was discharged from the hospital to home 10 days later. In the four months since his definitive repair, the patient has continued to do well and can now ambulate 2-3 city blocks without resting. Repeat TTE demonstrated no fistula, and his subjective symptoms of shortness of breath have resolved.
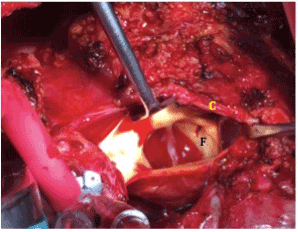
Figure 2.4x2 mm fistula (F) in right coronary Sinus of Valsalva just below ostia of right coronary sinus (C).
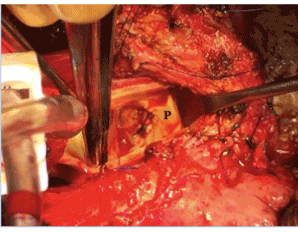
Figure 3. Patch closure of fistula (P).
Comment
Delayed cardiac injuries can be categorized into valvular injuries, aneurysms, retained foreign bodies, and, most commonly, shunts [3]. In a ten-year period at Inkosi Albert Luthuli Central Hospital in South Africa, 10 patients manifested delayed lesions requiring operative repair, half for ventricular septal defects. One patient required repair of an aorto-RV fistula with associated valvular regurgitation presenting three weeks post-injury with congestive heart failure [1].
Patients with traumatic aorto-RV fistulas will return with symptoms of congestive heart failure in 72% of cases [4]. Timing of delayed cardiac lesions is variable in the literature and ranges from 1 day to 33 years, with 80% presenting by four weeks [1]. Our patient had progressed to high output heart failure three weeks post-injury.
2021 Copyright OAT. All rights reserv
Therefore, importance lies in early identification of patients at risk for delayed complications. However, the optimal diagnostic modality, timing, and even mandate for routine testing remain controversial. If the patient’s hemodynamic status permits, intraoperative blood gas sampling can identify occult intracardiac shunts [5]. Post-operative TTE is a more commonly utilized modality, but it may have a limited yield as it can be difficult to identify the fistula. Early post-traumatic edema or clot can obfuscate defects and hemodynamic instability can affect the quality of imaging. Echocardiographic findings may not correlate with the patient’s clinical status.
Degiannis, et al. performed echocardiography on all survivors of penetrating cardiac injury at discharge, one-month, and three-month follow-up. One patient was found to have an abnormality at discharge, which was an asymptomatic valvular lesion that did not require operative intervention. No other intracardiac defects were found at one and three months [6].
We do not routinely perform screening TTE, but rather select patients based on clinical symptoms. In our case, the search for a source of bacteremia prompted TTE 10 days post-injury. If we performed TTE earlier, the small fistula may have been missed altogether. Conversely, it may have led to earlier repair before the window of re-do sternotomy closed. Surgical repair is indicated in all cases of aorto-RV fistulas. The defect is unlikely to spontaneously heal, due to injury involving the membranous septum [4].
It is unusual to repair a life-threatening, penetrating cardiac injury and successfully resuscitate a patient, only to be faced with a delayed traumatic, intracardiac complication such as a fistula requiring re-operative cardiac surgery. Immediate resuscitation of these patients is paramount and should follow established trauma algorithms. Early utilization of clinically specific modalities such as echocardiography can aid in discovery of secondary injury, but patient symptoms and trajectory of injury should also guide clinicians to suspect a possible intra-cardiac fistula. Finally, proper timing of definitive repair is paramount, and could be the difference between a young life saved or a courageous multidisciplinary effort all for naught.
Disclosures
None
References
- Reddy D, Muckart DJ (2014) Holes in the heart: An atlas of intracardiac injuries following penetrating trauma. Interac Cardiovas Thorac Surg 19:56-63. [Crossref]
- Campbell NC, Thomson SR, Muckart DJ, Meumann CM, Van Middelkoop I, et al. (1997) Review of 1198 cases of penetrating cardiac trauma. Br J Surg 84: 1737-1740. [Crossref]
- Beall AC, Ochsner JL, Morris GC, Cooley DA, Debakey ME (1961) Penetrating wounds of the heart. J Trauma 1: 195-207.
- Samuels LE, Kaufman MS, Rodriguez-Vega J, Morris RJ, Brockman SK (1998) Diagnosis and management of traumatic aorto-right ventricular fistulas. Ann Thorac Surg 65: 288-292. [Crossref]
- Baxter BT, Moore EE, Moore FA, Pomerantz M (1989) Intraoperative cardiac sampling following penetrating wounds: A technique for early detection of traumatic intracardiac shunts. J Trauma 29: 1719-1720. [Crossref]
- Degiannis E, Loogna P, Doll D, Bonnano F, Bowley DM, et al (2006) Penetrating cardiac injuries: Recent experience in South Africa. World J Surg 30:1258-1264. [Crossref]



