The manufacture of pills and tablets for human use worldwide is done under clean but non-aseptic conditions. Many of the drugs humans take today are little more than a gelatin capsule containing an active pharmaceutical agent (API). These simple drugs which cost pennies on the dollar to manufacture are often sold at hundreds of dollars a pill. The manufacturing conditions for these products are in confined spaces with limited airflow, and a great deal of industrial dust. Water and humidity are almost non-existent in these facilities which lead to an environment with a low bioburden. The absence of water and humidity are essential, if under-discussed components of solid oral drug manufacture. The presence of water leads to growth of bacteria and the absence of water will tend to retard the growth of bacteria, limiting the potential bioburden.
Water, Bacteria, Chemical, Capsule, Bioburden
Manufacturing of solid oral dosage formulations is by definition non-sterile. This is in contrast to parenterals which are by definition sterile upon completion of manufacturing. Water is the solvent in which proteins, polysaccharide and lipids are dissolved, but in the confines of solid oral manufacturing may be considered little more than a contaminant. Water may be taken into excipients, which comprise an individual part of the pill or capsule, but which are inert in terms of physiological function. The best, or most noticeable example of an excipient is the gelatin capsule. Gelatin is highly hydroscopic, and attracts water absorbed from the ambient environment. Capsules and pills are normally manufactured in the absence of any water thus limiting the presence of the molecule. The availability of water is the most important single factor affecting the growth of all prokaryotic and eukaryotic cells. The availability of water for a cell depends upon its presence in the atmosphere (relative humidity) or its presence in solution, or a substance. Water activity is affected by the presence of solutes such as salts and/or sugars that may be dissolved in a given solution. The lower the solute concentration of a substance, the higher the water activity.
Water activity has effective ranges where it may be compared to pure water as essentially a control. The water activity (Aw) of pure H2O is set at 1.0 as a standard (100% water) [1]. The Aw of most fresh vegetables is 0.99 or greater. The Aw of human blood is 0.99; seawater = 0.98; maple syrup = 0.90; Great Salt Lake = 0.75. Water activities in agricultural soils range between 0.9 and 1.0. Microorganisms will thrive over a range of Aw from 1.0 to 0.7. The proliferative activity of microorganisms is curtailed at 0.50 [2].
Water activity (Aw) may be defined as:
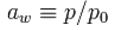
Where p is the vapor pressure of water in the substance, and p₀ is the vapor pressure of pure water at the same temperature.
The relative humidity of the manufacturing environment is important as products are affected by the amount of water in the atmosphere making it critical that water and relative humidity are monitored. Water activity is monitored in finished pharmaceutical products by analytical chemistry analysis. In facilities, it is monitored continuously by FDA Part 11 compliant computer systems
The relative humidity of air in equilibrium with a sample is called the Equilibrium Relative Humidity (ERH).
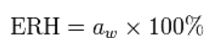
Facilities that manufacture solid oral dosage formulations are evaluated on a routine basis for relative humidity, cleanliness, particulate matter in the air and bioburden (microbial contamination) [3]. Bacteria, yeasts and molds are evaluated in the facility using basic microbiological technique, and by continually monitoring the facility especially for airborne particles and humidity. Cleaning is essential for controlling microbial growth, especially in geographic areas of higher humidity such as Puerto Rico and India where much pharmaceutical manufacturing is done [4].
In general, bacteria tend to require more available water for growth than do the yeasts or molds. Most bacteria require an Aw greater than 0.91 for growth while most molds can grow at Aw levels as low as 0.80 [5].
The concept of lowering water activity in order to prevent bacterial growth is the basis for preservation. Several methods are used but two of the most important are (1) drying, such as by evaporation or (2) by addition of high concentrations of salt or sugar thus affecting the solute to solvent ratio. Osmolarity is determined by solute concentration in the environment and is inversely related to water activity. Increased solute concentration means increased osmolarity and decreased Aw. In terms of microbial growth, the optimal growth rate of Salmonella, E. coli or Pseudomonas is in the range of 0.94 to 0.99 Aw. [6]. A lower Aw (0.85) is optimal for the growth rate of a halotolerant bacterium such as Staphylococcus aureus, and the growth rate of an extreme halophile such as is found in the Great Salt Lake may be as low as 0.7. A true halophile grows best at salt concentrations where most bacteria are killed or the growth rate is reduced [5].
Spore forming bacteria such as Bacillus sps. can survive extremes of water, variations in salt concentration and/or temperature extremes for extended periods of time. A spore is like a plant seed, which allows the plant to survive harsh conditions, such as reduced water levels. Interestingly, a newly formed Bacillus sps bacterial cell, which has yet to form a spore has an Aw of 0.90. Microbial proliferation should not occur below a water activity level of 0.50. There is simply not enough water to maintain cellular structure.
Solid oral dosage formulations are a dry blended product produced under non-aseptic manufacturing conditions that are absent of water. They are by definition non-sterile. The manufacturing areas are under conditions of low humidity and individual operators are gowned, usually wearing self-contained respirators or filter units to prevent the intake of toxic dust, and also to protect them from potential toxic products. Because of the absence of water and the separation (gowning) of the individual operators from product there is a substantially reduced risk of product contamination and thus growth of microorganisms associated with the product.
Two additional points should be made. First virus is not assayed for in these facilities. Whether this will be required by regulatory agencies at some point is an open question. Second, none of the water activity information relates to spore forming bacteria. This is the major issue in industrial settings because these organisms are designed (by evolution) to withstand the worst conditions including, desiccation. The great equalizer in all this is that the route of entry for these products is the oral cavity, with a final destination of the gut. Since the oral cavity and the gut are massively contaminated, primarily with E. coli, there is little danger in most normal heathy patients that an infection will occur, as the rest of the body is effectively ‘walled off” from these organisms.
- Mossel DAA, Corry JEL, Struijk CB, Baird RM 1995. Essentials of the microbiology of foods: a textbook for advanced studies. Chichester (England): John Wiley and Sons. 699 p.
- John Lindquist. Based on material in the Laboratory Manual for the Food Microbiology Laboratory (1998 edition, edited by John L. and published at the University) which was used in Bacteriology/Food Science 324.
- Marczynski Z, Zgoda M, Jambor J (2007) Application of silicified microcrystalline cellulose (Prosolv) as a polymer carrier of Epilobium parviflorun Schreb. Extract in oral solid drug form. Polim Med 37: 21-32. [Crossref]
- Vesper S, Choi H, Perzanowski MS, Acosta LM, Divjan A, et al. (2016) Mold populations and dust mite allergen concentrations in house dust samples from across Puerto Rico. Int J Environ Health Res 26: 198-207. [Crossref]
- United States Pharmacopeia 32, Chapter 1112.
- Farakos S, Hicks J, Frye J, Frank JF (2014) Relative survival of four serotypes of Salmonella enterica in low-water activity whey protein powder held at 36 and 70 ºC at various water activity levels. J Food Prot 77: 1198-2000. [Crossref]
2021 Copyright OAT. All rights reserv
